Clin Exp Otorhinolaryngol.
2015 Jun;8(2):83-91. 10.3342/ceo.2015.8.2.83.
Neural-Induced Human Mesenchymal Stem Cells Promote Cochlear Cell Regeneration in Deaf Guinea Pigs
- Affiliations
-
- 1Department of Physiology, Chonnam National University Medical School, Gwangju, Korea.
- 2The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Korea.
- 3Research Institute of Medical Sciences, Chonnam National University, Gwangju, Korea. choyb@chonnam.ac.kr
- 4Department of Otolaryngology-Head and Neck Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 5Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- 6Department of Physiology, Chosun University College of Medicine, Gwangju, Korea.
- KMID: 2360776
- DOI: http://doi.org/10.3342/ceo.2015.8.2.83
Abstract
OBJECTIVES
In mammals, cochlear hair cell loss is irreversible and may result in a permanent sensorineural hearing loss. Secondary to this hair cell loss, a progressive loss of spiral ganglion neurons (SGNs) is presented. In this study, we have investigated the effects of neural-induced human mesenchymal stem cells (NI-hMSCs) from human bone marrow on sensory neuronal regeneration from neomycin treated deafened guinea pig cochleae.
METHODS
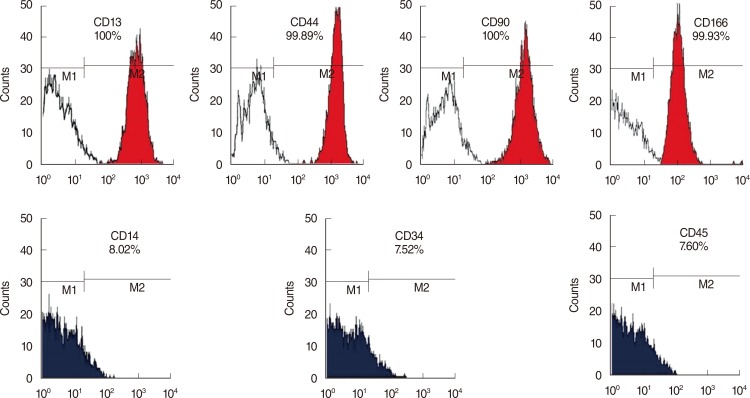
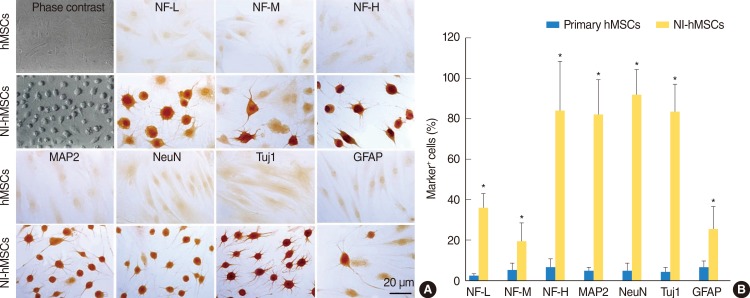
HMSCs were isolated from the bone marrow which was obtained from the mastoid process during mastoidectomy for ear surgery. Following neural induction with basic fibroblast growth factor and forskolin, we studied the several neural marker and performed electrophysiological analysis. NI-hMSCs were transplanted into the neomycin treated deafened guinea pig cochlea. Engraftment of NI-hMSCs was evaluated immunohistologically at 8 weeks after transplantation.
RESULTS
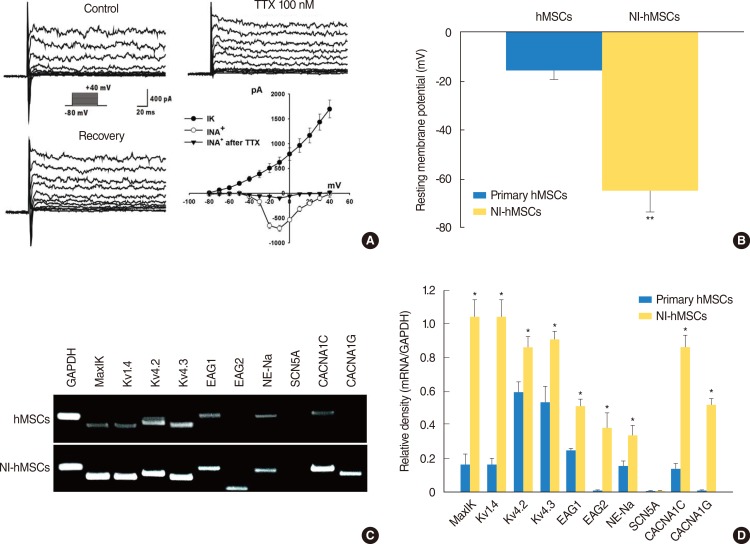
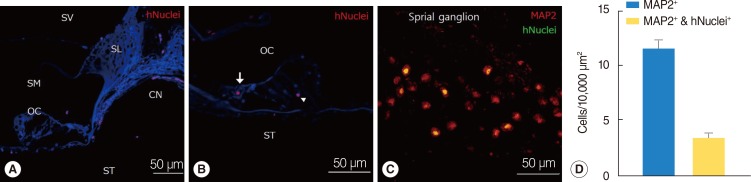
Following neural differentiation, hMSCs expressed high levels of neural markers, ionic channel markers, which are important in neural function, and tetrodotoxin-sensitive voltage-dependent sodium currents. After transplantation into the scala tympani of damaged cochlea, NI-hMSCs-injected animals exhibited a significant increase in the number of SGNs compared to Hanks balanced salt solution-injected animals. Transplanted NI-hMSCs were found within the perilymphatic space, the organ of Corti, along the cochlear nerve fibers, and in the spiral ganglion. Furthermore, the grafted NI-hMSCs migrated into the spiral ganglion where they expressed the neuron-specific marker, NeuN.
CONCLUSION
The results show the potential of NI-hMSCs to give rise to replace the lost cochlear cells in hearing loss mammals.
Keyword
MeSH Terms
-
Animals
Bone Marrow
Cell Differentiation
Cochlea
Cochlear Nerve
Colforsin
Ear
Fibroblast Growth Factor 2
Guinea Pigs*
Hair
Hearing Loss
Hearing Loss, Sensorineural
Humans
Ion Channels
Mammals
Mastoid
Mesenchymal Stromal Cells*
Neomycin
Neurons
Organ of Corti
Regeneration*
Scala Tympani
Sensory Receptor Cells
Sodium
Spiral Ganglion
Transplantation
Transplants
Colforsin
Fibroblast Growth Factor 2
Ion Channels
Neomycin
Sodium
Figure
Reference
-
1. Holley MC. Keynote review: the auditory system, hearing loss and potential targets for drug development. Drug Discov Today. 2005; 10. 10(19):1269–1282. PMID: 16214671.
Article2. Warchol ME, Lambert PR, Goldstein BJ, Forge A, Corwin JT. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993; 3. 259(5101):1619–1622. PMID: 8456285.
Article3. Ernfors P, Kucera J, Lee KF, Loring J, Jaenisch R. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995; 10. 39(5):799–807. PMID: 8645564.4. Tan J, Shepherd RK. Aminoglycoside-induced degeneration of adult spiral ganglion neurons involves differential modulation of tyrosine kinase B and p75 neurotrophin receptor signaling. Am J Pathol. 2006; 8. 169(2):528–543. PMID: 16877354.
Article5. Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000; 6. 97(13):7597–7602. PMID: 10861021.
Article6. Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005; 3. 11(3):271–276. PMID: 15711559.
Article7. Sage C, Huang M, Karimi K, Gutierrez G, Vollrath MA, Zhang DS, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005; 2. 307(5712):1114–1118. PMID: 15653467.
Article8. Li H, Roblin G, Liu H, Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc Natl Acad Sci U S A. 2003; 11. 100(23):13495–13500. PMID: 14593207.
Article10. Kim SS, Yoo SW, Park TS, Ahn SC, Jeong HS, Kim JW, et al. Neural induction with neurogenin1 increases the therapeutic effects of mesenchymal stem cells in the ischemic brain. Stem Cells. 2008; 9. 26(9):2217–2228. PMID: 18617687.
Article11. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000; 11. 6(11):1282–1286. PMID: 11062543.
Article12. Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004; 6. 113(12):1701–1710. PMID: 15199405.
Article13. Chen W, Jongkamonwiwat N, Abbas L, Eshtan SJ, Johnson SL, Kuhn S, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012; 10. 490(7419):278–282. PMID: 22972191.
Article14. Parker MA, Corliss DA, Gray B, Anderson JK, Bobbin RP, Snyder EY, et al. Neural stem cells injected into the sound-damaged cochlea migrate throughout the cochlea and express markers of hair cells, supporting cells, and spiral ganglion cells. Hear Res. 2007; 10. 232(1-2):29–43. PMID: 17659854.
Article15. Revoltella RP, Papini S, Rosellini A, Michelini M, Franceschini V, Ciorba A, et al. Cochlear repair by transplantation of human cord blood CD133+ cells to nod-scid mice made deaf with kanamycin and noise. Cell Transplant. 2008; 17(6):665–678. PMID: 18819255.
Article16. Nishimura K, Nakagawa T, Ono K, Ogita H, Sakamoto T, Yamamoto N, et al. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009; 9. 20(14):1250–1254. PMID: 19625987.
Article17. Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003; 10. 9(10):1293–1299. PMID: 12949502.
Article18. Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010; 4. 11:25. PMID: 20398362.
Article19. Cho YB, Cho HH, Jang S, Jeong HS, Park JS. Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model. J Korean Med Sci. 2011; 4. 26(4):492–498. PMID: 21468255.
Article20. Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007; 13(1):119–126. PMID: 17266591.
Article21. Jiang Y, Henderson D, Blackstad M, Chen A, Miller RF, Verfaillie CM. Neuroectodermal differentiation from mouse multipotent adult progenitor cells. Proc Natl Acad Sci U S A. 2003; 9. 100(Suppl 1):11854–11860. PMID: 12925733.
Article22. Anghileri E, Marconi S, Pignatelli A, Cifelli P, Galie M, Sbarbati A, et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008; 10. 17(5):909–916. PMID: 18564036.
Article23. Li GR, Deng XL, Sun H, Chung SS, Tse HF, Lau CP. Ion channels in mesenchymal stem cells from rat bone marrow. Stem Cells. 2006; 6. 24(6):1519–1528. PMID: 16484345.
Article24. Sharif S, Nakagawa T, Ohno T, Matsumoto M, Kita T, Riazuddin S, et al. The potential use of bone marrow stromal cells for cochlear cell therapy. Neuroreport. 2007; 3. 18(4):351–354. PMID: 17435601.
Article25. Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007; 7. 171(1):214–226. PMID: 17591967.
Article26. Matsuoka AJ, Kondo T, Miyamoto RT, Hashino E. Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy. Laryngoscope. 2007; 9. 117(9):1629–1635. PMID: 17632425.
Article27. Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, et al. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005; 1. 302(1):40–47. PMID: 15541724.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Bone Regeneration from Adipose Stem Cells
- Transplantation of Neural Differentiated Human Mesenchymal Stem Cells into the Cochlea of an Auditory-neuropathy Guinea Pig Model
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Isolation, Characterization and Growth Kinetic Comparison of Bone Marrow and Adipose Tissue Mesenchymal Stem Cells of Guinea Pig