Clin Exp Otorhinolaryngol.
2016 Dec;9(4):358-365. 10.21053/ceo.2015.01508.
Effectiveness of Palatal Mucosa Graft in Surgical Treatment of Sub-Glottic Stenosis
- Affiliations
-
- 1Department of Thoracic Surgery, Reconstructive and Aesthetic Surgery, Medical Faculty of Pamukkale University, Denizli, Turkey. mdaydogmus@yahoo.com
- 2Department of Plastic, Reconstructive and Aesthetic Surgery, Medical Faculty of Pamukkale University, Denizli, Turkey.
- 3Department of Pathology, Medical Faculty of Pamukkale University, Denizli, Turkey.
- 4Department of Experimental Research Laboratory, Medical Faculty of Pamukkale University, Denizli, Turkey.
- 5Department of Thoracic Surgery, Liv Hospital, Istanbul, Turkey.
- KMID: 2360768
- DOI: http://doi.org/10.21053/ceo.2015.01508
Abstract
OBJECTIVES
Mucosal free grafts may be successfully applied in many surgical interventions. This study aims at investigating the feasibility of palatal mucosa graft in sub-glottic field in an animal model.
METHODS
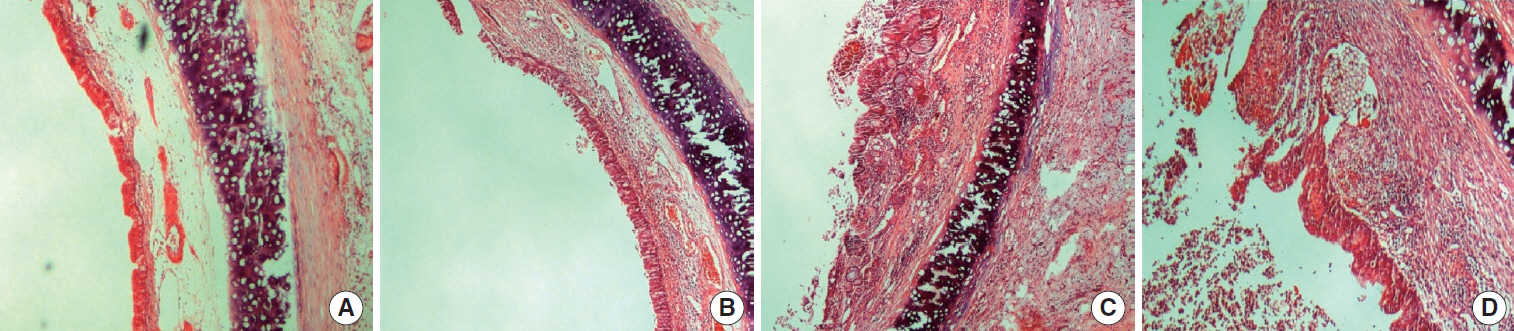
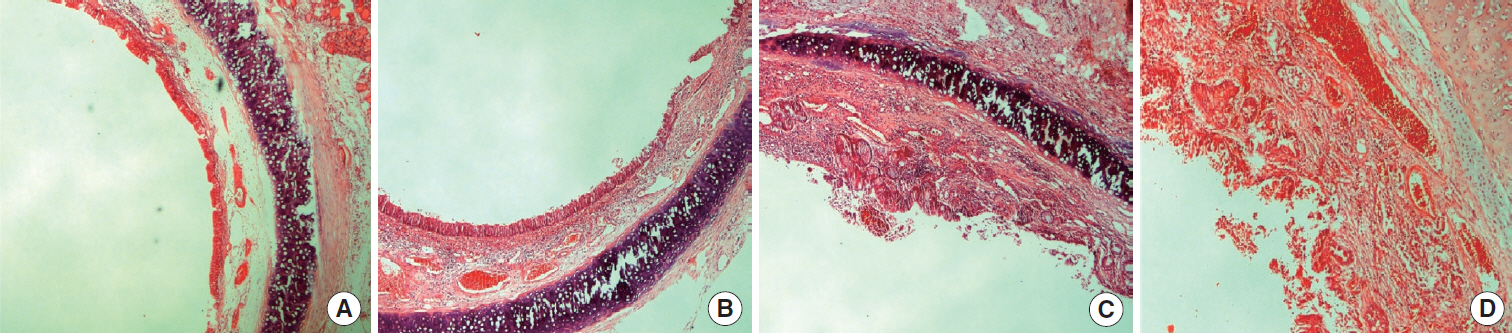
This randomized prospective controlled study was conducted with an animal model. Sub-glottic inflammation was created in 15 adult rabbits in each group and sub-glottic stenosis surgery was applied thereafter. The rabbits in group 1 (control group) underwent segmental resection, partial cricoidectomy, and trachea-thyroid cartilage anastomosis; the rabbits in group 2 underwent segmental resection, cricoplasty, and crico-tracheal anastomosis using free buccal mucosa graft; and the rabbits in group 3 underwent segmental resection, cricoplasty, and crico-tracheal anastomosis using free palatal mucosa graft. Re-stenosis was evaluated after 42 days.
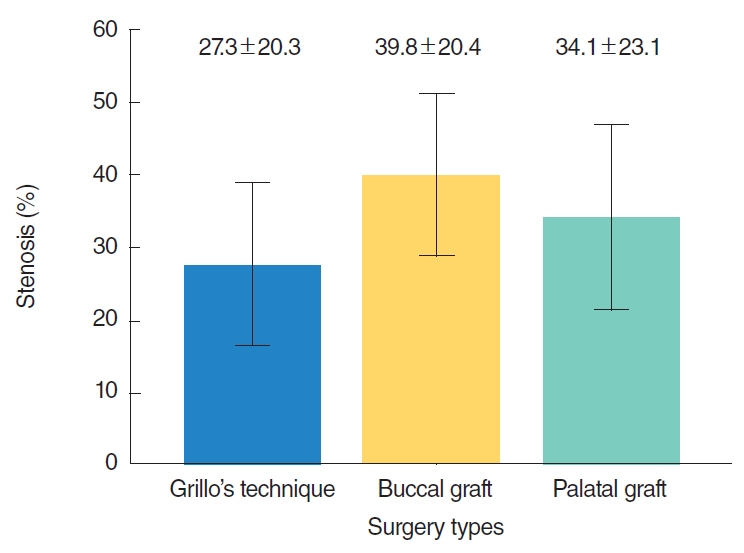
RESULTS
The percentages of stenosis were 27%±20%, 40%±20%, and 34%±23% for group 1, 2, and 3, respectively and the difference was not statistically significant (P=0.29). Intensive and tight fibrosis was observed in 2 rabbits (13%) in group 1, in 5 rabbits (33%) in group 2, and in 3 rabbits (20%) in group 3. There was not a statistically significant difference between groups (P=0.41). Excessive inflammation was observed in 3 rabbits (20%) in group 1, in 7 rabbits (47%) in group 2, and 3 rabbits (20%) in group 3. There was no a statistically significant difference between groups although inflammation rate was higher in the rabbits which underwent buccal mucosa graft (P=0.18).
CONCLUSION
The surgical treatments applied with free mucosa graft reduced anastomosis tension through enabling anastomosis to the distal of cricoid instead of thyroid cartilage. Free palatal mucosa grafts may be used in sub-glottic field, one of the most challenging fields of trachea surgery, due to ease of application and rapid vascularization.
Keyword
MeSH Terms
Figure
Reference
-
1. Haft S, Lee JY, Ghosh A, Philiponis G, Malaisrie N, Leahy KP, et al. Inflammatory protein expression in human subglottic stenosis tissue mirrors that in a murine model. Ann Otol Rhinol Laryngol. 2014; Jan. 123(1):65–70.
Article2. Rubikas R, Matukaitytė I, Jelisiejevas JJ, Rackauskas M. Surgical treatment of non-malignant laryngotracheal stenosis. Eur Arch Otorhinolaryngol. 2014; Sep. 271(9):2481–7.
Article3. Gomez-Caro A, Morcillo A, Wins R, Molins L, Galan G, Tarrazona V. Surgical management of benign tracheal stenosis. Multimed Man Cardiothorac Surg. 2011; Jan. 2011(1111):mmcts.2010.004945.
Article4. Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg. 1982; Jan. 33(1):3–18.
Article5. Montgomery WW. Chronic subglottic stenosis. Otolaryngol Clin North Am. 1984; Feb. 17(1):107–13.
Article6. Liberman M, Mathisen DJ. Tailored cricoplasty: an improved modification for reconstruction in subglottic tracheal stenosis. J Thorac Cardiovasc Surg. 2009; Mar. 137(3):573–8.
Article7. Holck DE, Foster JA, Dutton JJ, Dillon HD. Hard palate mucosal grafts in the treatment of the contracted socket. Ophthal Plast Reconstr Surg. 1999; May. 15(3):202–9.8. Myer CM 3rd, O’Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol. 1994; Apr. 103(4 Pt 1):319–23.
Article9. Iniguez-Cuadra R, San Martin Prieto J, Iniguez-Cuadra M, Zuniga Erranz S, Jofre Pavez D, Gonzalez Bombardiere S, et al. Effect of mitomycin in the surgical treatment of tracheal stenosis. Arch Otolaryngol Head Neck Surg. 2008; Jul. 134(7):709–14.
Article10. Couraud L, Jougon JB, Velly JF. Surgical treatment of nontumoral stenoses of the upper airway. Ann Thorac Surg. 1995; Aug. 60(2):250–9.
Article11. Pearson FG. Technique of management of subglottic stenosis. Chest Surg Clin N Am. 1996; Nov. 6(4):683–92.12. Macchiarini P, Verhoye JP, Chapelier A, Fadel E, Dartevelle P. Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation subglottic stenosis. J Thorac Cardiovasc Surg. 2001; Jan. 121(1):68–76.
Article13. Aydogmus U, Turk F, Yuncu G. Surgical repair with palatal mucosal graft in subglottic stenosis caused by Wegener’s granulomatosis. Turk Gogus Kalp Damar Cerrahisi Dergisi. 2015; 23(3):595–600.14. Hatoko M, Tanaka A, Kuwahara M, Yurugi S, Niitsuma K, Iioka H. Influence of periosteum on donor healing after harvesting hard palate mucosa. Ann Plast Surg. 2003; Jan. 50(1):25–30.
Article15. Cohen MS, Shorr N. Eyelid reconstruction with hard palate mucosa grafts. Ophthal Plast Reconstr Surg. 1992; Sep. 8(3):183–95.
Article16. Yamamoto K, Kojima F, Tomiyama K, Nakamura T, Hayashino Y. Meta-analysis of therapeutic procedures for acquired subglottic stenosis in adults. Ann Thorac Surg. 2011; Jun. 91(6):1747–53.
Article17. Wright CD, Grillo HC, Wain JC, Wong DR, Donahue DM, Gaissert HA, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg. 2004; Nov. 128(5):731–9.
Article18. Montgomery WW. Suprahyoid release for tracheal anastomosis. Arch Otolaryngol. 1974; Apr. 99(4):255–60.
Article19. Haykal S, Salna M, Waddell TK, Hofer SO. Advances in tracheal reconstruction. Plast Reconstr Surg Glob Open. 2014; Aug. 2(7):e178.
Article20. Neville WE, Bolanowski PJ, Soltanzadeh H. Homograft replacement of the trachea using immunosuppression. J Thorac Cardiovasc Surg. 1976; Oct. 72(4):596–601.
Article21. Weisberger EC, Nguyen CT. Laryngotracheal reconstruction using a Vitallium alloy miniplate. Ann Otol Rhinol Laryngol. 1996; May. 105(5):363–6.
Article22. Carron JD, Greinwald JH, Oberman JP, Werner AL, Derkay CS. Simulated reflux and laryngotracheal reconstruction: a rabbit model. Arch Otolaryngol Head Neck Surg. 2001; May. 127(5):576–80.23. De Jong AL, Park AH, Raveh E, Schwartz MR, Forte V. Comparison of thyroid, auricular, and costal cartilage donor sites for laryngotracheal reconstruction in an animal model. Arch Otolaryngol Head Neck Surg. 2000; Jan. 126(1):49–53.
Article24. Hirata T, Yamazaki F, Fukuse T, Muro K, Yokomise H, Inui K, Takahashi Y, et al. Omentopexy for revascularization of free tracheal grafts in rats. Thorac Cardiovasc Surg. 1992; Aug. 40(4):178–81.
Article25. Kumaran S, Nambi GI, Kingsly Paul M, Gupta AK. Post-electrical-burn tracheal-defect reconstruction with pre-fabricated deltopectoral flap: a case report. J Plast Reconstr Aesthet Surg. 2009; May. 62(5):e93–4.26. He J, Xu X, Chen M, Li S, Yin W, Wang S, et al. Novel method to repair tracheal defect by pectoralis major myocutaneous flap. Ann Thorac Surg. 2009; Jul. 88(1):288–91.
Article27. Masuda M, Kamizono K, Ejima M, Fujimura A, Uryu H, Kadota H. Tracheal reconstruction with a modified infrahyoid myocutaneous flap. Laryngoscope. 2012; May. 122(5):992–6.
Article28. Tan Q, Steiner R, Hoerstrup SP, Weder W. Tissue-engineered trachea: History, problems and the future. Eur J Cardiothorac Surg. 2006; Nov. 30(5):782–6.
Article29. Shin YS, Lee BH, Choi JW, Min BH, Chang JW, Yang SS, et al. Tissue-engineered tracheal reconstruction using chondrocyte seeded on a porcine cartilage-derived substance scaffold. Int J Pediatr Otorhinolaryngol. 2014; Jan. 78(1):32–8.
Article30. Wright CD, Graham BB, Grillo HC, Wain JC, Mathisen DJ. Pediatric tracheal surgery. Ann Thorac Surg. 2002; Aug. 74(2):308–13.
Article31. Montgomery WW. T-tube tracheal stent. Arch Otolaryngol. 1965; Sep. 82(3):320–1.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CO2 Laser Microsurgery for Type 1 Posterior Glottic Stenosis Misdiagnosed as Bronchial Asthma: A Case Report
- Free Gingival Graft to Gain Peri-implant Keratinized Mucosa
- Relationship between The Shape of Palatal Vault and Tooth and The Thickness of Palatal Masticatory Mucosa
- A Comparative study on the palatal mucosa thickness measurements using periodontal probe and pltrasonic device
- Voice and Videostroboscopic Analysis of Sulcus Vocalis after Slicing Mucosa Surgical Technique