J Korean Med Sci.
2016 Feb;31(2):275-279. 10.3346/jkms.2016.31.2.275.
Estrogen-mediated Height Control in Girls with Marfan Syndrome
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. dooseok.choi@samsung.com
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2360053
- DOI: http://doi.org/10.3346/jkms.2016.31.2.275
Abstract
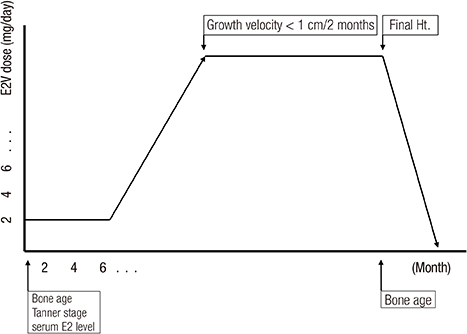
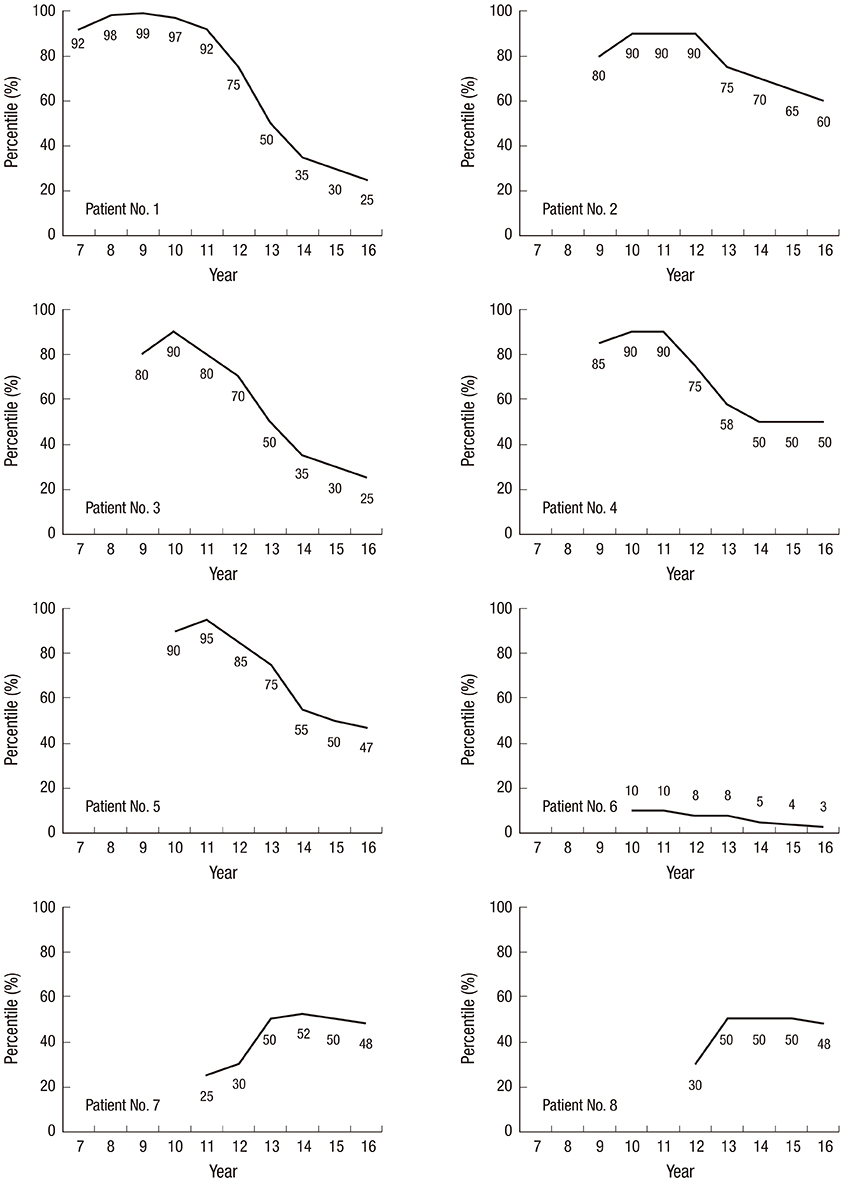
- This study evaluated the efficacy of a stepwise regimen of estradiol valerate for height control in girls with Marfan syndrome. Eight girls with Marfan syndrome who had completed estrogen treatment for height control were included. Estradiol valerate was started at a dose of 2 mg/day, and then was increased. The projected final height was estimated using the initial height percentile (on a disease-specific growth curve for Korean Marfan syndrome [gcPFHt]), and the initial bone age (baPFHt). After the estrogen treatment, the projected final height was compared to the actual final height (FHt). The median baseline chronological and bone age were 10.0 and 10.5 years, respectively. After a median of 36.5 months of treatment, the median FHt (172.6 cm) was shorter than the median gcPFHt (181.0 cm) and baPFHt (175.9 cm). In the six patients who started treatment before the age of 11 years, the median FHt (171.8 cm) was shorter than the median gcPFHt (181.5 cm) and baPFHt (177.4 cm) after treatment. The median differences between the FHt and gcPFHt and baPFHt were 9.2 and 8.3 cm, respectively. In two patients started treatment after the age of 11, the differences between FHt and gcPFHt, and baPFHt after treatment were -4 and 1.4 cm, and -1.2 and 0 cm for each case, respectively. A stepwise increasing regimen of estradiol valerate may be an effective treatment for height control in girls with Marfan syndrome, especially when started under 11 years old.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical Characteristics of Gynecologic Problems During Childhood in the Korean Population
Haewon Choi, Sung Eun Kim, Nae Hyun Lee, Dong-Yun Lee, DooSeok Choi
J Korean Med Sci. 2023;38(37):e279. doi: 10.3346/jkms.2023.38.e279.
Reference
-
1. Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005; 366:1965–1976.2. Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991; 352:337–339.3. Erkula G, Jones KB, Sponseller PD, Dietz HC, Pyeritz RE. Growth and maturation in Marfan syndrome. Am J Med Genet. 2002; 109:100–115.4. Vetter U, Mayerhofer R, Lang D, von Bernuth G, Ranke MB, Schmaltz AA. The Marfan syndrome--analysis of growth and cardiovascular manifestation. Eur J Pediatr. 1990; 149:452–456.5. Joseph KN, Kane HA, Milner RS, Steg NL, Williamson MB Jr, Bowen JR. Orthopedic aspects of the Marfan phenotype. Clin Orthop Relat Res. 1992; (277):251–261.6. Drop SL, De Waal WJ, De Muinck Keizer-Schrama SM. Sex steroid treatment of constitutionally tall stature. Endocr Rev. 1998; 19:540–558.7. de Waal WJ, Greyn-Fokker MH, Stijnen T, van Gurp EA, Toolens AM, de Munick Keizer-Schrama SM, Aarsen RS, Drop SL. Accuracy of final height prediction and effect of growth-reductive therapy in 362 constitutionally tall children. J Clin Endocrinol Metab. 1996; 81:1206–1216.8. Reeser HM, Heremans GF, van Gelderen HH. Reduction of adult height in tall girls. Eur J Pediatr. 1979; 132:37–41.9. Skovby F, McKusick VA. Estrogen treatment of tall stature in girls with the Marfan syndrome. Birth Defects Orig Artic Ser. 1977; 13:155–161.10. Knudtzon J, Aarskog D. Estrogen treatment of excessively tall girls with Marfan syndrome. Acta Paediatr Scand. 1988; 77:537–541.11. Rozendaal L, le Cessie S, Wit JM, Hennekam RC. Dutch Marfan Working Group. Growth-reductive therapy in children with marfan syndrome. J Pediatr. 2005; 147:674–679.12. Ucar SK, Paterson WF, Donaldson MD, Young D. Ethinyl estradiol treatment for growth limitation in girls with Marfan’s syndrome--experience from a single center. Endocr Res. 2009; 34:109–120.13. Borgelt LM, Martell CW. Estradiol valerate/dienogest: a novel combined oral contraceptive. Clin Ther. 2012; 34:37–55.14. De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996; 62:417–426.15. Kwun Y, Kim SJ, Lee J, Isojima T, Choi DS, Kim DK, Huh J, Kang IS, Chang M, Cho SY, et al. Disease-specific growth charts of Marfan syndrome patients in Korea. J Korean Med Sci. 2015; 30:911–916.16. Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch Dis Child. 1976; 51:170–179.17. Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist based on the brush foundation study of human growth and development initiated by T. Wingate Todd, M.B., Ch.B., F.R.C.S. JAMA. 1950; 143:1124.18. Kuhn N, Blunck W, Stahnke N, Wiebel J, Willig RP. Estrogen treatment in tall girls. Acta Paediatr Scand. 1977; 66:161–167.19. Wiedemann E, Schwartz E. Suppression of growth hormone-dependent human serum sulfation factor by estrogen. J Clin Endocrinol. 1972; 34:51–58.20. Bailey JD, Park E, Cowell C. Estrogen treatment of girls and constitutional tall stature. Pediatr Clin North Am. 1981; 28:501–512.21. Lyon AJ, Preece MA, Grant DB. Growth curve for girls with Turner syndrome. Arch Dis Child. 1985; 60:932–935.