J Korean Med Sci.
2015 Dec;30(12):1777-1783. 10.3346/jkms.2015.30.12.1777.
Comparison of HE4, CA125, and Risk of Ovarian Malignancy Algorithm in the Prediction of Ovarian Cancer in Korean Women
- Affiliations
-
- 1Department of Obstetrics & Gynecology, Hallym University Medical Center, Hwaseong, Korea. msfeel@hallym.or.kr
- 2Department of Obstetrics & Gynecology, Chungbuk National University College of Medicine, Cheongju, Korea.
- KMID: 2359961
- DOI: http://doi.org/10.3346/jkms.2015.30.12.1777
Abstract
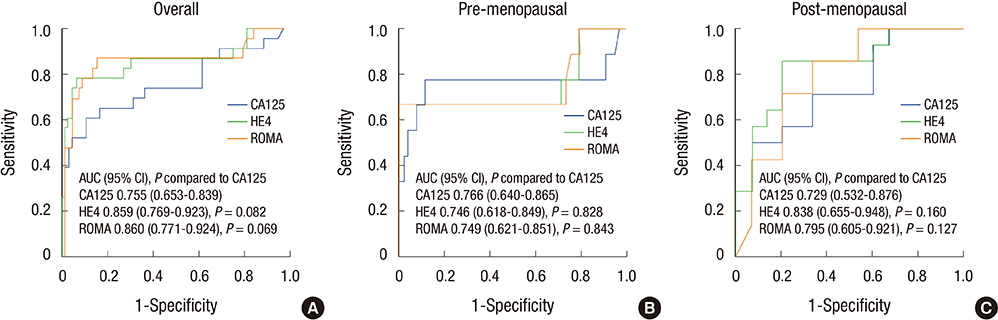
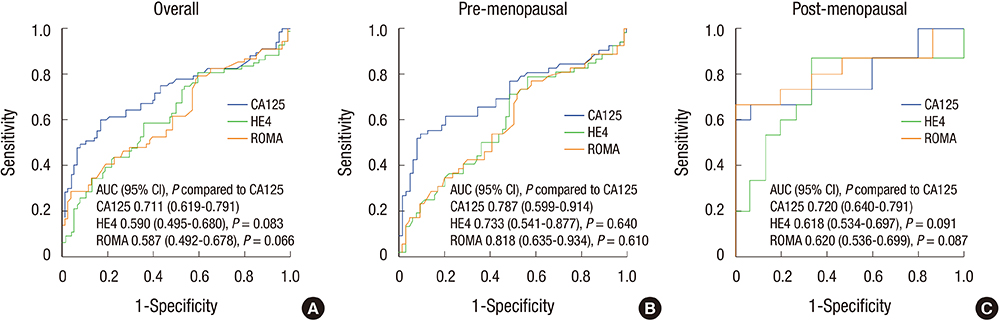
- This study is a multi-center clinical study, which aimed to compare CA125, HE4, and risk of ovarian malignancy algorithm (ROMA) in predicting epithelial ovarian cancer of Korean women with a pelvic mass. Prospectively, serum from 90 Korean women with ovarian mass was obtained prior to surgery. For control group, serum from 79 normal populations without ovarian mass was also obtained. The HE4 and CA125 data were registered and evaluated separately and ROMA was calculated for each sample. Total 67 benign tumors and 23 ovarian cancers were evaluated. Median serum levels of HE4 and CA125, and ROMA score were significantly higher in patients with ovarian cancer than those with benign ovarian tumor and normal population (P < 0.001). In ROC curve analysis for women with a pelvic mass, area under the curve (AUC) for HE4 and ROMA was higher than CA125. Statistical differences in each study compared to CA125 were marginal (P compared to CA125; 0.082 for HE4 and 0.069 for ROMA). Sub-analysis revealed that AUC for HE4 and ROMA was higher than AUC for CA125 in post-menopausal women with a pelvic mass, but there were no statistically significant differences (P compared to CA125; 0.160 for HE4 and 0.127 for ROMA). Our data suggested that both HE4 and ROMA score showed better performance than CA125 for the detection of ovarian cancer in women with a pelvic mass. HE4 and ROMA can be a useful independent diagnostic marker for epithelial ovarian cancer in Korean women.
Keyword
MeSH Terms
-
Algorithms
Area Under Curve
Biomarkers, Tumor/blood
CA-125 Antigen/*blood
Case-Control Studies
Female
Humans
Middle Aged
Neoplasms, Glandular and Epithelial/*blood/*diagnosis
Ovarian Neoplasms/*blood/*diagnosis
Predictive Value of Tests
Prospective Studies
Proteins/*metabolism
ROC Curve
Reference Values
Republic of Korea
Biomarkers, Tumor
CA-125 Antigen
Proteins
Figure
Cited by 2 articles
-
The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4
Kyung Hee Han, Noh Hyun Park, Jin Ju Kim, Sunmie Kim, Hee Seung Kim, Maria Lee, Yong Sang Song
J Gynecol Oncol. 2019;30(6):. doi: 10.3802/jgo.2019.30.e83.Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
Kyung Nam Kang, Eun Young Koh, Ji Young Jang, Chul Woo Kim
Obstet Gynecol Sci. 2022;65(4):346-354. doi: 10.5468/ogs.22017.
Reference
-
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.2. Barakat RR, Markman M, Randall ME. Principles and practice of gynecologic oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins;2009. p. 757–847.3. Mehta DA, Hay JW. Cost-effectiveness of adding bevacizumab to first line therapy for patients with advanced ovarian cancer. Gynecol Oncol. 2014; 132:677–683.4. Havrilesky LJ, Pokrzywinski R, Revicki D, Higgins RV, Nycum LR, Kohler MF, Berchuck A, Myers ER, Secord AA. Cost-effectiveness of combination versus sequential docetaxel and carboplatin for the treatment of platinum-sensitive, recurrent ovarian cancer. Cancer. 2012; 118:386–391.5. Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med. 2009; 361:170–177.6. Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011; 305:2295–2303.7. Gilbert L, Basso O, Sampalis J, Karp I, Martins C, Feng J, Piedimonte S, Quintal L, Ramanakumar AV, Takefman J, et al. Assessment of symptomatic women for early diagnosis of ovarian cancer: results from the prospective DOvE pilot project. Lancet Oncol. 2012; 13:285–291.8. Valentin L, Jurkovic D, Van Calster B, Testa A, Van Holsbeke C, Bourne T, Vergote I, Van Huffel S, Timmerman D. Adding a single CA 125 measurement to ultrasound imaging performed by an experienced examiner does not improve preoperative discrimination between benign and malignant adnexal masses. Ultrasound Obstet Gynecol. 2009; 34:345–354.9. Håkansson F, Høgdall EV, Nedergaard L, Lundvall L, Engelholm SA, Pedersen AT, Hartwell D, Høgdall C. Danish 'pelvic mass' ovarian cancer study. Risk of malignancy index used as a diagnostic tool in a tertiary centre for patients with a pelvic mass. Acta Obstet Gynecol Scand. 2012; 91:496–502.10. van den Akker PA, Aalders AL, Snijders MP, Kluivers KB, Samlal RA, Vollebergh JH, Massuger LF. Evaluation of the Risk of Malignancy Index in daily clinical management of adnexal masses. Gynecol Oncol. 2010; 116:384–388.11. Duffy MJ, Bonfrer JM, Kulpa J, Rustin GJ, Soletormos G, Torre GC, Tuxen MK, Zwirner M. CA125 in ovarian cancer: European Group on Tumor Markers guidelines for clinical use. Int J Gynecol Cancer. 2005; 15:679–691.12. Rosen DG, Wang L, Atkinson JN, Yu Y, Lu KH, Diamandis EP, Hellstrom I, Mok SC, Liu J, Bast RC Jr. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol Oncol. 2005; 99:267–277.13. Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005; 65:2162–2169.14. Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellström KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003; 63:3695–3700.15. Holcomb K, Vucetic Z, Miller MC, Knapp RC. Human epididymis protein 4 offers superior specificity in the differentiation of benign and malignant adnexal masses in premenopausal women. Am J Obstet Gynecol. 2011; 205:358.e1–358.e6.16. Redman C, Duffy S, Bromham N, Francis K. Guideline Development Group. Recognition and initial management of ovarian cancer: summary of NICE guidance. BMJ. 2011; 342:d2073.17. Andersen MR, Goff BA, Lowe KA, Scholler N, Bergan L, Drescher CW, Paley P, Urban N. Use of a Symptom Index, CA125, and HE4 to predict ovarian cancer. Gynecol Oncol. 2010; 116:378–383.18. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC Jr, Skates SJ. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009; 112:40–46.19. Lenhard M, Stieber P, Hertlein L, Kirschenhofer A, Fürst S, Mayr D, Nagel D, Hofmann K, Krocker K, Burges A. The diagnostic accuracy of two human epididymis protein 4 (HE4) testing systems in combination with CA125 in the differential diagnosis of ovarian masses. Clin Chem Lab Med. 2011; 49:2081–2088.20. Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC, Skates SJ. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010; 203:228.e1–228.e6.21. Molina R, Escudero JM, Augé JM, Filella X, Foj L, Torné A, Lejarcegui J, Pahisa J. HE4 a novel tumour marker for ovarian cancer: comparison with CA 125 and ROMA algorithm in patients with gynaecological diseases. Tumour Biol. 2011; 32:1087–1095.22. Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, Timmerman D, De Moor B, Vergote I. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011; 104:863–870.23. Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, Fink D, Heinzelmann-Schwarz V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011; 121:487–491.24. Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, Sabaratnam S, Hebbar S, Sickan J, Schodin BA, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013; 128:239–244.25. Fujiwara H, Suzuki M, Takeshima N, Takizawa K, Kimura E, Nakanishi T, Yamada K, Takano H, Sasaki H, Koyama K, et al. Evaluation of human epididymis protein 4 (HE4) and Risk of Ovarian Malignancy Algorithm (ROMA) as diagnostic tools of type I and type II epithelial ovarian cancer in Japanese women. Tumour Biol. 2015; 36:1045–1053.26. Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989; 96:889–892.27. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008; 108:402–408.28. Wu L, Dai ZY, Qian YH, Shi Y, Liu FJ, Yang C. Diagnostic value of serum human epididymis protein 4 (HE4) in ovarian carcinoma: a systematic review and meta-analysis. Int J Gynecol Cancer. 2012; 22:1106–1112.29. Shah CA, Lowe KA, Paley P, Wallace E, Anderson GL, McIntosh MW, Andersen MR, Scholler N, Bergan LA, Thorpe JD, et al. Influence of ovarian cancer risk status on the diagnostic performance of the serum biomarkers mesothelin, HE4, and CA125. Cancer Epidemiol Biomarkers Prev. 2009; 18:1365–1372.30. Montagnana M, Lippi G, Ruzzenente O, Bresciani V, Danese E, Scevarolli S, Salvagno GL, Giudici S, Franchi M, Guidi GC. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal. 2009; 23:331–335.31. Wilailak S, Chan KK, Chen CA, Nam JH, Ochiai K, Aw TC, Sabaratnam S, Hebbar S, Sickan J, Schodin BA, et al. Distinguishing benign from malignant pelvic mass utilizing an algorithm with HE4, menopausal status, and ultrasound findings. J Gynecol Oncol. 2015; 26:46–53.32. Chung SH, Lee SY, Ju W, Kim SC. Clinical efficacy of serum human epididymis protein 4 as a diagnostic biomarker of ovarian cancer: A pilot study. Obstet Gynecol Sci. 2013; 56:234–241.33. Ruggeri G, Bandiera E, Zanotti L, Belloli S, Ravaggi A, Romani C, Bignotti E, Tassi RA, Tognon G, Galli C, et al. HE4 and epithelial ovarian cancer: comparison and clinical evaluation of two immunoassays and a combination algorithm. Clin Chim Acta. 2011; 412:1447–1453.34. Sandri MT, Bottari F, Franchi D, Boveri S, Candiani M, Ronzoni S, Peiretti M, Radice D, Passerini R, Sideri M. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol Oncol. 2013; 128:233–238.35. Braicu EI, Van Gorp T, Nassir M, Richter R, Chekerov R, Gasimli K, Timmerman D, Vergote I, Sehouli J. Preoperative HE4 and ROMA values do not improve the CA125 diagnostic value for borderline tumors of the ovary (BOT) - a study of the TOC Consortium. J Ovarian Res. 2014; 7:49.36. Tung KH, Goodman MT, Wu AH, McDuffie K, Wilkens LR, Kolonel LN, Nomura AM, Terada KY, Carney ME, Sobin LH. Reproductive factors and epithelial ovarian cancer risk by histologic type: a multiethnic case-control study. Am J Epidemiol. 2003; 158:629–638.37. Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC Jr, Lambert-Messerlian G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am J Obstet Gynecol. 2012; 206:349.e1–349.e7.38. Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, Ha M, Choi KH. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. Clin Chem Lab Med. 2011; 49:527–534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epididymis Protein 4 as a Diagnostic Marker of Ovarian Cancer and Its Reference Interval in Korean Population
- Reference Range of HE4 in Healthy Women: Analytical Performance and Correlation with CA125
- Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- Clinical Usefulness of Cancer Antigen (CA) 125, Human Epididymis 4, and CA72-4 Levels and Risk of Ovarian Malignancy Algorithm Values for Diagnosing Ovarian Tumors in Korean Patients With and Without Endometriosis
- The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4