Ann Lab Med.
2020 Jan;40(1):40-47. 10.3343/alm.2020.40.1.40.
Clinical Usefulness of Cancer Antigen (CA) 125, Human Epididymis 4, and CA72-4 Levels and Risk of Ovarian Malignancy Algorithm Values for Diagnosing Ovarian Tumors in Korean Patients With and Without Endometriosis
- Affiliations
-
- 1Department of Laboratory Medicine and Biomedical Research Institute, Pusan National University Hospital and Pusan National University School of Medicine, Busan, Korea. hhkim@pusan.ac.kr
- 2Department of Obstetrics and Gynecology, Pusan National University School of Medicine, Busan, Korea.
- 3Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2457492
- DOI: http://doi.org/10.3343/alm.2020.40.1.40
Abstract
- BACKGROUND
Tumor markers are useful for detection and preoperative evaluation of ovarian tumors. We evaluated the clinical usefulness of cancer antigen (CA) 125, human epididymis 4 (HE4), and CA72-4 levels and Risk of Ovarian Malignancy Algorithm (ROMA) values for differential diagnosis of malignant and borderline tumors among suspected ovarian tumors, and the effects of endometriosis on these tumor markers.
METHODS
In a total of 266 patients (213, 14, and 39 with benign, borderline and malignant tumors, respectively), CA125, HE4, and CA72-4 levels were measured, and ROMA values were calculated. Medians of each marker were compared among the three groups. The area under the ROC curve (AUC), sensitivity, and specificity were calculated to analyze the diagnostic performance of each marker.
RESULTS
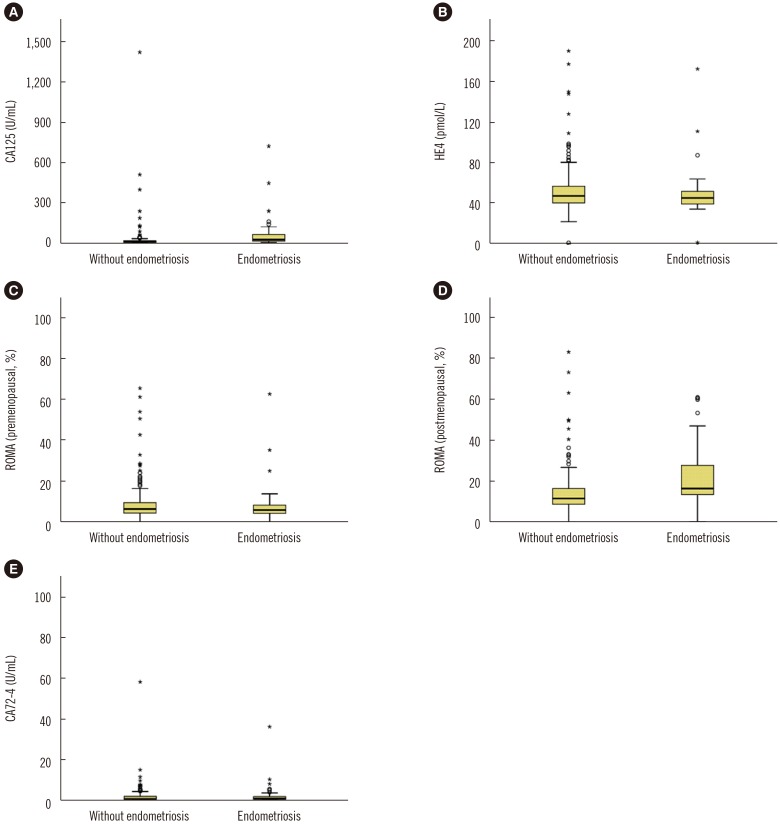
All markers were significantly higher in the malignant group than in the benign group. HE4 levels and ROMA values were significantly higher in the malignant group than in the borderline group. ROMA value had the highest AUC for distinguishing the malignant and borderline groups from the benign group in premenopausal (0.773) and postmenopausal (0.927) patients. CA125 level was significantly higher in patients with endometriosis than in those without (P<0.001), whereas HE4 and CA72-4 levels were not affected by endometriosis (P=0.128 and 0.271, respectively).
CONCLUSIONS
ROMA value is the best marker to distinguish malignant and borderline tumors from benign tumors in pre- and postmenopausal patients. HE4 and CA72-4 levels provide information on possible CA125 elevation due to endometriosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424. PMID: 30207593.2. Tchagang AB, Tewfik AH, DeRycke MS, Skubitz KM, Skubitz AP. Early detection of ovarian cancer using group biomarkers. Mol Cancer Ther. 2008; 7:27–37. PMID: 18187805.3. Daly MB, Pilarski R, Berry MP, Buys SS, Friedman S, Garber JE, et al. NCCN Guidelines Insights: genetic/familial high-risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017; 15:9–20. PMID: 28040716.4. Fotopoulou C, Hall M, Cruickshank D, Gabra H, Ganesan R, Hughes C, et al. British Gynaecological Cancer Society (BGCS) epithelial ovarian/Fallopian tube/primary peritoneal cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2017; 213:123–139. PMID: 28457647.5. Committee on Gynecologic Practice, Society of Gynecologic Oncology. Committee Opinion No. 716. Committee Opinion No. 716: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer in women at average risk. Obstet Gynecol. 2017; 130:e146–e149. PMID: 28832487.6. Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011; 57:1534–1544. PMID: 21933899.7. Aggarwal P, Kehoe SJM. Serum tumour markers in gynaecological cancers. Maturitas. 2010; 67:46–53. PMID: 20510555.8. Schummer M, Ng WV, Bumgarner RE, Nelson PS, Schummer B, Bednarski DW, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene. 1999; 238:375–385. PMID: 10570965.9. Guadagni F, Roselli M, Cosimelli M, Ferroni P, Spila A, Cavaliere F, et al. CA 72-4 serum marker—a new tool in the management of carcinoma patients. Cancer Invest. 1995; 13:227–238. PMID: 7874576.10. Lenhard MS, Nehring S, Nagel D, Mayr D, Kirschenhofer A, Hertlein L, et al. Predictive value of CA 125 and CA 72-4 in ovarian borderline tumors. Clin Chem Lab Med. 2009; 47:537–542. PMID: 19317653.11. Granato T, Midulla C, Longo F, Colaprisca B, Frati L, Anastasi E. Role of HE4, CA72.4, and CA125 in monitoring ovarian cancer. Tumour Biol. 2012; 33:1335–1339. PMID: 22528938.12. Yang WL, Gentry-Maharaj A, Simmons A, Ryan A, Fourkala EO, Lu Z, et al. Elevation of TP53 autoantibody before CA125 in preclinical invasive epithelial ovarian cancer. Clin Cancer Res. 2017; 23:5912–5922. PMID: 28637689.13. Chudecka-Głaz AM. ROMA, an algorithm for ovarian cancer. Clin Chim Acta. 2015; 440:143–151. PMID: 25447706.14. Prat J. FIGO Committee on Gynecologic Oncology. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. J Gynecol Oncol. 2015; 26:87–89. PMID: 25872889.15. Moore RG, Miller MC, Disilvestro P, Landrum LM, Gajewski W, Ball JJ, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011; 118:280–288. PMID: 21775843.16. Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013; 66:273–281. PMID: 23426716.17. Wang J, Gao J, Yao H, Wu Z, Wang M, Qi J. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol. 2014; 35:6127–6138. PMID: 24627132.18. Scaletta G, Plotti F, Luvero D, Capriglione S, Montera R, Miranda A, et al. The role of novel biomarker HE4 in the diagnosis, prognosis and follow-up of ovarian cancer: a systematic review. Expert Rev Anticancer Ther. 2017; 17:827–839. PMID: 28756722.19. Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009; 112:40–46. PMID: 18851871.20. Bandiera E, Romani C, Specchia C, Zanotti L, Galli C, Ruggeri G, et al. Serum human epididymis protein 4 and risk for ovarian malignancy algorithm as new diagnostic and prognostic tools for epithelial ovarian management. Cancer Epidemiol Biomarkers Prev. 2011; 20:2496–2506. PMID: 22028406.21. Ławicki S, Będkowska GE, Gacuta-Szumarska E, Szmitkowski M. The plasma concentration of VEGF, HE4 and CA125 as a new biomarkers panel in different stages and sub-types of epithelial ovarian tumors. J Ovarian Res. 2013; 6:45. PMID: 23819707.22. Van Gorp T, Cadron I, Despierre E, Daemen A, Leunen K, Amant F, et al. HE4 and CA125 as a diagnostic test in ovarian cancer: prospective validation of the Risk of Ovarian Malignancy Algorithm. Br J Cancer. 2011; 104:863–870. PMID: 21304524.23. Moore RG, Miller MC, Steinhoff MM, Skates SJ, Lu KH, Lambert-Messerlian G, et al. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2012; 206:351.e1–351.e8. PMID: 22284961.24. Anastasi E, Granato T, Falzarano R, Storelli P, Ticino A, Frati L, et al. The use of HE4, CA125 and CA72-4 biomarkers for differential diagnosis between ovarian endometrioma and epithelial ovarian cancer. J Ovarian Res. 2013; 6:44. PMID: 23816286.25. Nagy B Jr, Krasznai ZT, Balla H, Csobán M, Antal-Szalmás P, Hernádi Z, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012; 49:377–380. PMID: 22688735.26. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008; 108:402–408. PMID: 18061248.27. Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, et al. No benefit from combining HE4 and CA 125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011; 121:487–491. PMID: 21420727.28. Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013; 128:239–244. PMID: 23063998.29. Kadija S, Stefanovic A, Jeremic K, Radojevic MM, Nikolic L, Markovic I, et al. The utility of human epididymal protein 4, cancer antigen 125, and risk for malignancy algorithm in ovarian cancer and endometriosis. Int J Gynecol Cancer. 2012; 22:238–244. PMID: 22214964.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epididymis Protein 4 as a Diagnostic Marker of Ovarian Cancer and Its Reference Interval in Korean Population
- Clinical efficacy of serum human epididymis protein 4 as a diagnostic biomarker of ovarian cancer: A pilot study
- A clinical evaluation of CA 125 antigen values in patients of ovarian cancer
- The power of the Risk of Ovarian Malignancy Algorithm considering menopausal status: a comparison with CA 125 and HE4
- Clinical application of serum CA 125 and CA 19-9 in Endometriosis