Obstet Gynecol Sci.
2013 Jul;56(4):234-241.
Clinical efficacy of serum human epididymis protein 4 as a diagnostic biomarker of ovarian cancer: A pilot study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University Mokdong Hospital, Ewha Womans University School of Medicine, Seoul, Korea. onco@ewha.ac.kr
- 2Department of Obstetrics and Gynecology, Cheil General Hospital and Women's Healthcare Center, Kwandong University College of Medicine, Seoul, Korea.
Abstract
OBJECTIVE
To compare accuracy of serum human epididymis protein 4 (HE4) levels with cancer antigen 125 (CA-125) levels as biomarkers for ovarian cancer.
METHODS
The study population included 94 Korean women, including 32 patients with a diagnosis of ovarian cancer and 62 patients with a diagnosis of benign ovarian tumor. All diagnoses were confirmed by histopathological analysis. Serum HE4 levels were assessed using an HE4 enzyme immunoassay, which were performed according to the manufacturer's instructions. Serum CA-125 levels were determined using a Modular analytics E170 module.
RESULTS
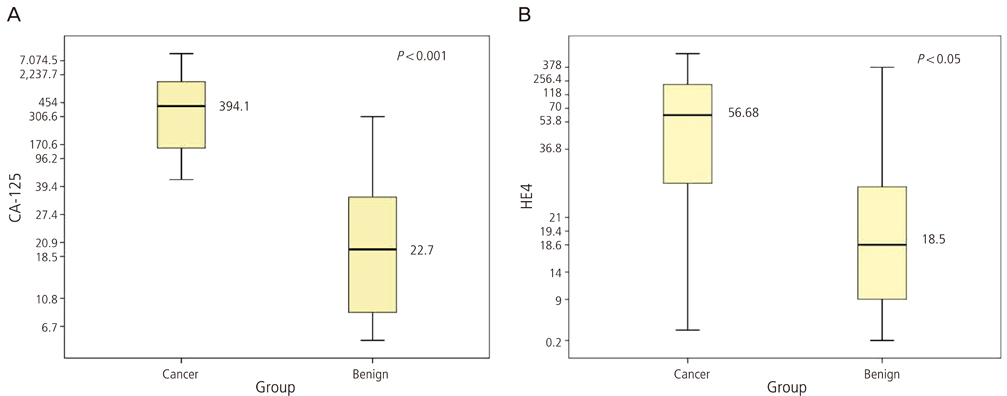
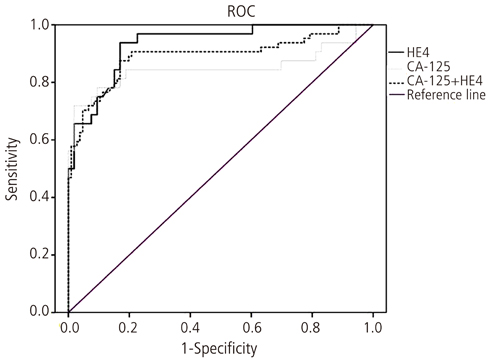
The median serum CA-125 and HE4 levels were significantly higher in patients with ovarian cancer than those with other benign tumors (CA-125, 394.1 U/mL vs. 22.7 U/mL; HE4, 56.7 pM vs. 18.5 pM; P < 0.05 in both). An additional comparison revealed that the patients with endometriosis had greater median serum CA-125 levels than those with other benign ovarian tumors (32.0 U/mL vs. 17.9 U/mL, P = 0.03). Conversely, the median serum HE4 levels were similar among the two benign ovarian tumor groups, with no statistically significant difference observed (19.0 pM vs. 18.2 pM, P = 0.49). The receiver operating characteristics curve analysis for the benign ovarian tumor and ovarian cancer patients showed that HE4 showed a greater area under the curve with borderline significance when compared with CA-125 in both groups (0.93 vs. 0.85).
CONCLUSION
Serum HE4 levels may not only allow for the detection of ovarian cancer, but also allow for better differentiation of cases of ovarian cancer versus other benign ovarian tumors compared with serum CA-125.
Keyword
MeSH Terms
Figure
Reference
-
1. Ozols RF. Recurrent ovarian cancer: evidence-based treatment. J Clin Oncol. 2002. 20:1161–1163.2. Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999. 80:827–841.3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.4. Park B, Park S, Kim TJ, Ma SH, Kim BG, Kim YM, et al. Epidemiological characteristics of ovarian cancer in Korea. J Gynecol Oncol. 2010. 21:241–247.5. National Cancer Information Center. Cancer incidence trend analysis 1999-2007 [Internet]. c2012. cited 2013 Jun 14. Goyang (KR): National Cancer Information Center;Available from: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer_040102000000.6. Surveillance epidemiology and end results. Ovary-cancer statistics review [Internet]. cited 2013 Jun 14. Bethesda (MD): National Cancer Institute;Available from: http://seer.cancer.gov/csr/1975_2009_pops09/results_merged/sect_21_ovary.pdf.7. Medeiros LR, Rosa DD, da Rosa MI, Bozzetti MC. Accuracy of CA 125 in the diagnosis of ovarian tumors: a quantitative systematic review. Eur J Obstet Gynecol Reprod Biol. 2009. 142:99–105.8. Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer. 2009. 115:1395–1403.9. Urban N, McIntosh MW, Andersen M, Karlan BY. Ovarian cancer screening. Hematol Oncol Clin North Am. 2003. 17:989–1005.10. Chen DX, Schwartz PE, Li XG, Yang Z. Evaluation of CA 125 levels in differentiating malignant from benign tumors in patients with pelvic masses. Obstet Gynecol. 1988. 72:23–27.11. Bast RC Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983. 309:883–887.12. Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989. 4:1–12.13. Kirchhoff C. Molecular characterization of epididymal proteins. Rev Reprod. 1998. 3:86–95.14. Clauss A, Lilja H, Lundwall A. A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein. Biochem J. 2002. 368:233–242.15. Bingle L, Singleton V, Bingle CD. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene. 2002. 21:2768–2773.16. Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003. 63:3695–3700.17. Galgano MT, Hampton GM, Frierson HF Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006. 19:847–853.18. Montagnana M, Danese E, Ruzzenente O, Bresciani V, Nuzzo T, Gelati M, et al. The ROMA (Risk of Ovarian Malignancy Algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med. 2011. 49:521–525.19. Abdel-Azeez HA, Labib HA, Sharaf SM, Refai AN. HE4 and mesothelin: novel biomarkers of ovarian carcinoma in patients with pelvic masses. Asian Pac J Cancer Prev. 2010. 11:111–116.20. Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008. 110:374–382.21. Rein BJ, Gupta S, Dada R, Safi J, Michener C, Agarwal A. Potential markers for detection and monitoring of ovarian cancer. J Oncol. 2011. 2011:475983.22. Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008. 108:402–408.23. Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009. 100:1315–1319.24. Partheen K, Kristjansdottir B, Sundfeldt K. Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass. J Gynecol Oncol. 2011. 22:244–252.25. Bae DH, Jeong HK, Kim MK, Seo SH, Shin YW, Cho TS. A comparative study between pulsatility index, new scoring system and CA-125 in detecting malignant ovarian tumor. Korean J Gynecol Oncol Colposc. 1993. 4:32–39.26. Nam JH, Park MC, Chung JK, Park SY, Lee Jh, Mok JE. Preoperative tumor marker levels in differentiating malignant from benign tumors in with pelvic masses. Korean J Gynecol Oncol Colposc. 1992. 3:1–9.27. Hwang J, Na S, Lee H, Lee D. Correlation between preoperative serum levels of five biomarkers and relationships between these biomarkers and cancer stage in epithelial overian cancer. J Gynecol Oncol. 2009. 20:169–175.28. Kim YM, Whang DH, Park J, Kim SH, Lee SW, Park HA, et al. Evaluation of the accuracy of serum human epididymis protein 4 in combination with CA125 for detecting ovarian cancer: a prospective case-control study in a Korean population. Clin Chem Lab Med. 2011. 49:527–534.29. Park Y, Kim Y, Lee EY, Lee JH, Kim HS. Reference ranges for HE4 and CA125 in a large Asian population by automated assays and diagnostic performances for ovarian cancer. Int J Cancer. 2012. 130:1136–1144.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Human Epididymis Protein 4 as a Diagnostic Marker of Ovarian Cancer and Its Reference Interval in Korean Population
- Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening
- Production and Performance Evaluation of External Quality Assessment Materials for Human Epididymis Protein 4 Assay
- Clinical Usefulness of Human Epididymis Protein 4 in Lung Cancer
- Reference Range of HE4 in Healthy Women: Analytical Performance and Correlation with CA125