Cancer Res Treat.
2016 Oct;48(4):1351-1362. 10.4143/crt.2015.444.
Survival Outcome of Combined GnRH Agonist and Tamoxifen Is Comparable to That of Sequential Adriamycin and Cyclophosphamide Chemotherapy Plus Tamoxifen in Premenopausal Patients with Lymph-Node–Negative, Hormone-Responsive, HER2-Negative, T1-T2 Breast Cancer
- Affiliations
-
- 1Division of Breast and Endocrine Surgery, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. ahnsh@amc.seoul.kr
- 2Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2356237
- DOI: http://doi.org/10.4143/crt.2015.444
Abstract
- PURPOSE
The purpose of this study was to compare treatment outcomes between combined gonadotropin-releasing hormone agonist and tamoxifen (GnRHa+T) and sequential adriamycin and cyclophosphamide chemotherapy and tamoxifen (AC->T) in premenopausal patients with hormone-responsive, lymph-node-negative breast cancer.
MATERIALS AND METHODS
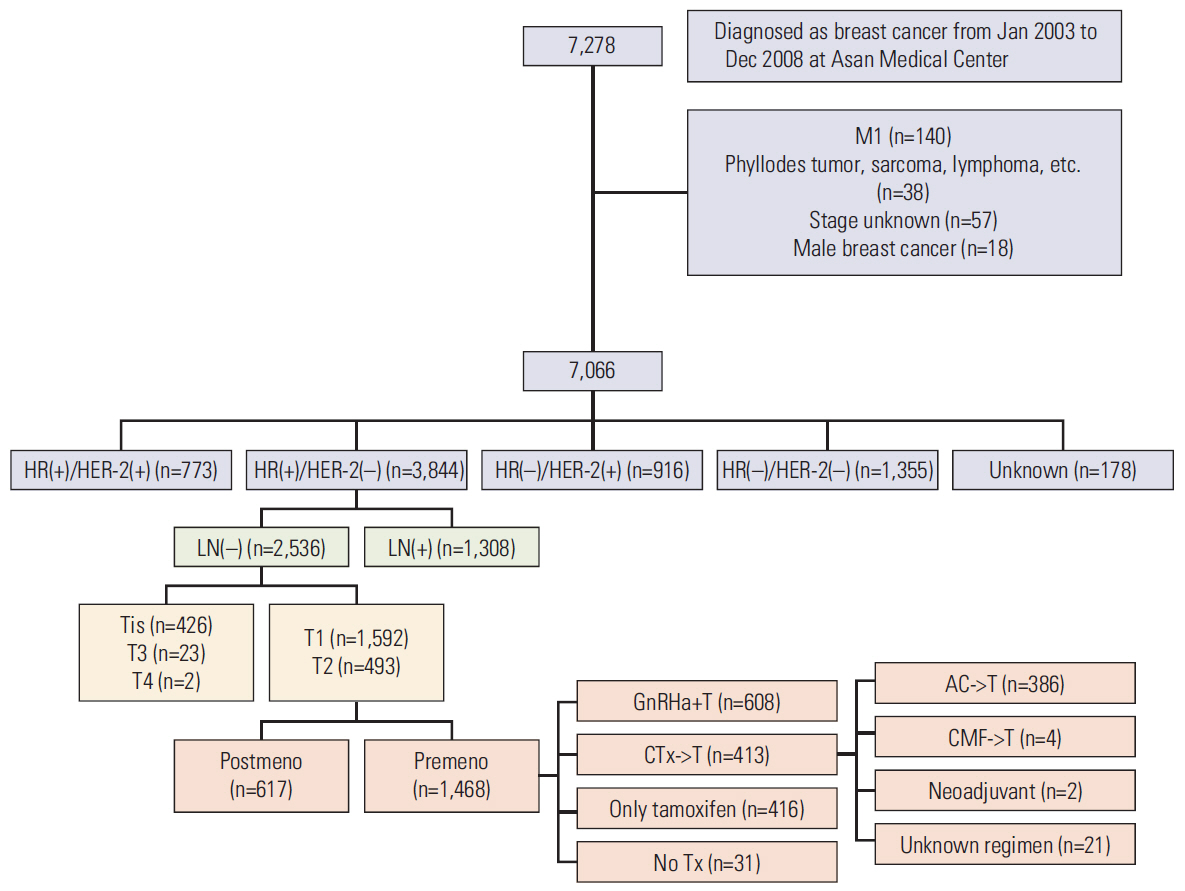
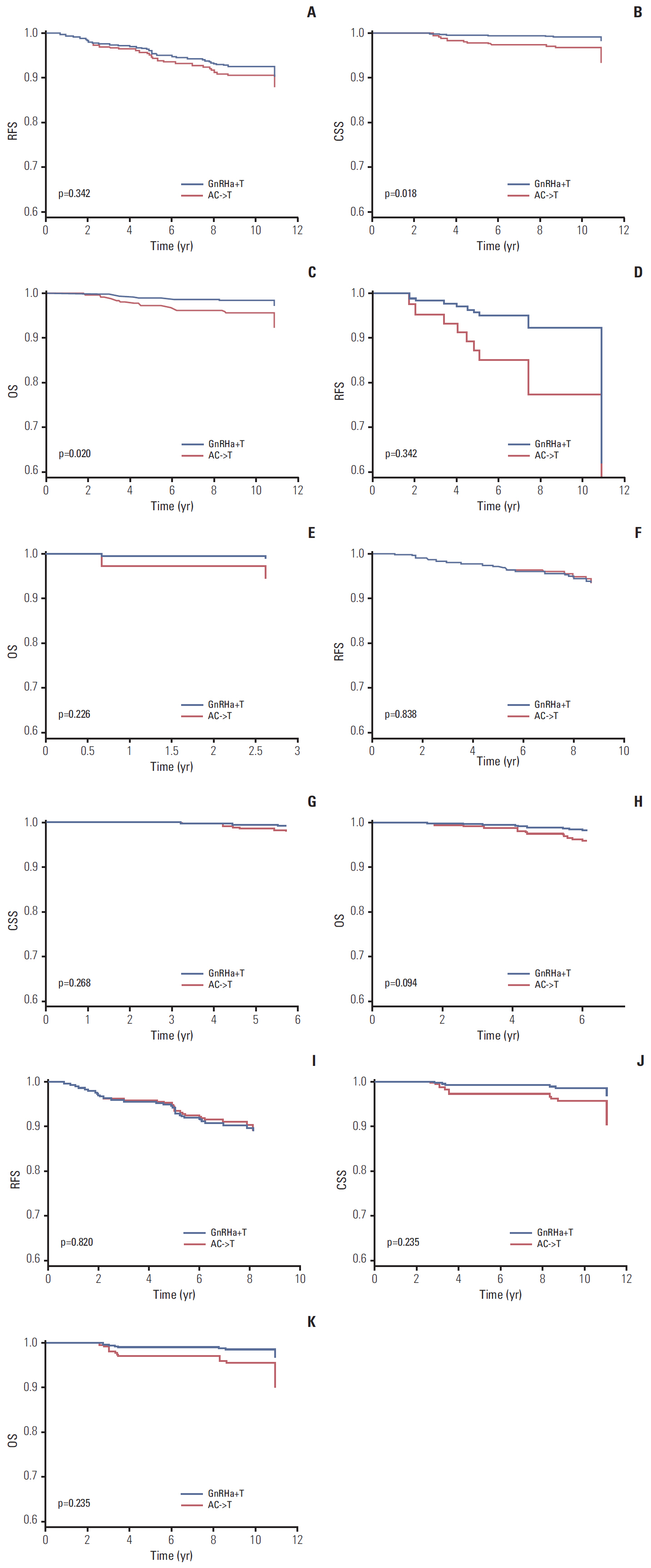
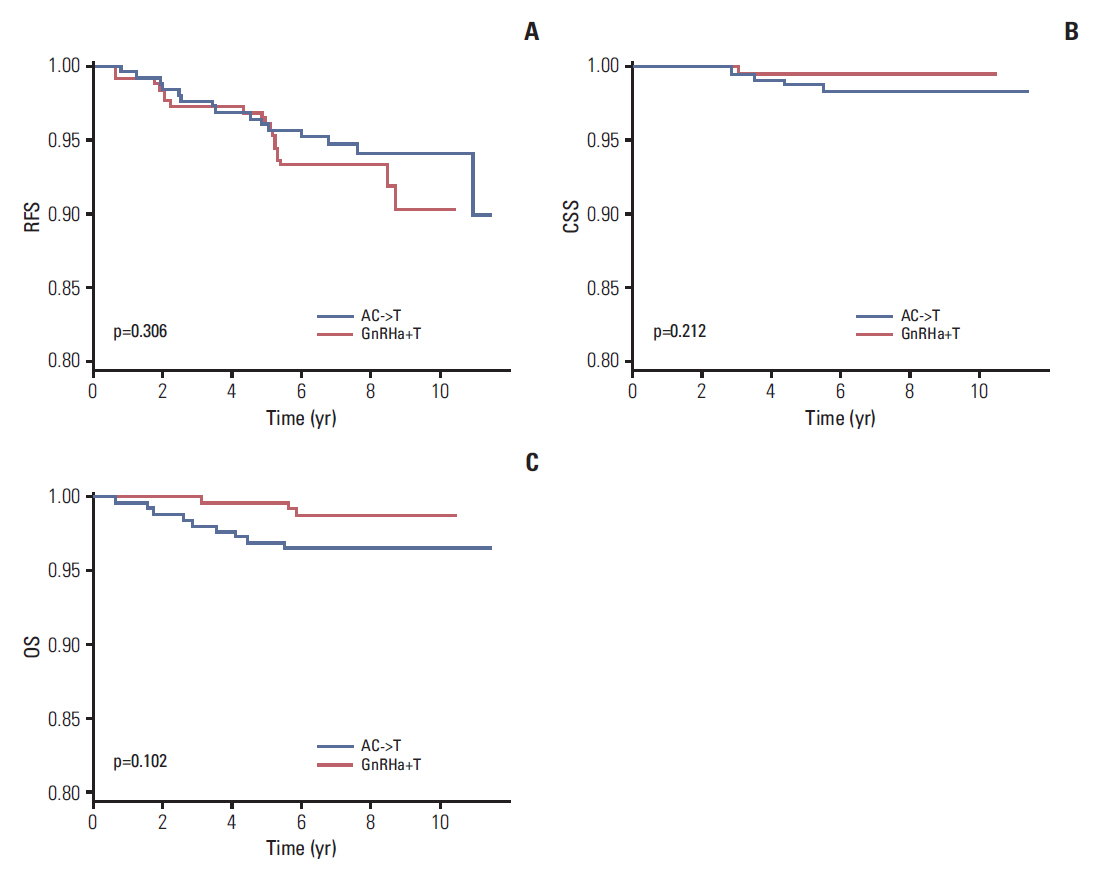
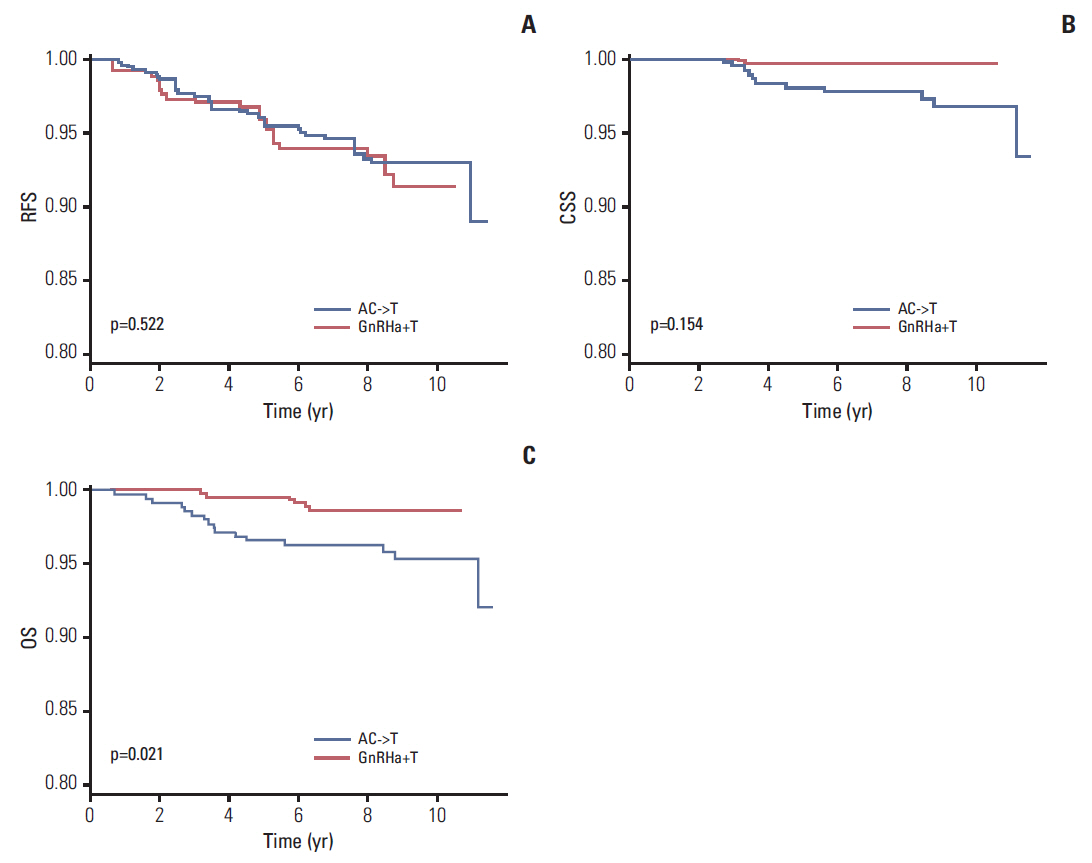
In total, 994 premenopausal women with T1-T2, lymph-node-negative, hormone-receptor-positive, HER2-negative breast cancer between January 2003 and December 2008 were included in this retrospective cohort study. GnRHa+T and AC->T were administered to 608 patients (61.2%) and 386 patients (38.8%), respectively. Propensity score matching and inverse probability weighting were applied to the original cohort, and 260 patients for each treatment arm were included in the final analysis. Recurrence-free, cancer-specific, and overall survival was compared between the two treatment groups.
RESULTS
A total of 994 patients were followed up for a median of 7.4 years (range, 0.5 to 11.4 years). The 5-year follow-up rate was 98.7%, and 13 patients were lost to follow-up. In propensity-matched cohorts (n=520), there was no difference in recurrence-free, cancer-specific, and overall survival rates between the two treatment groups (p=0.306, p=0.212, and p=0.102, respectively), and this was maintained after applying inverse probability weighting.
CONCLUSION
GnRHa+T is a reasonable alternative to AC->T in patients with premenopausal, hormone-responsive, HER2-negative, lymph-node-negative, T1-T2 breast cancer.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Schmid P, Untch M, Kosse V, Bondar G, Vassiljev L, Tarutinov V, et al. Leuprorelin acetate every-3-months depot versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant treatment in premenopausal patients with node-positive breast cancer: the TABLE study. J Clin Oncol. 2007; 25:2509–15.
Article2. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.
Article3. Francis PA, Regan MM, Fleming GF. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015; 372:1673.
Article4. Adjuvant ovarian ablation versus CMF chemotherapy in premenopausal women with pathological stage II breast carcinoma: the Scottish trial. Scottish Cancer Trials Breast Group and ICRF Breast Unit, Guy's Hospital, London. Lancet. 1993; 341:1293–8.5. Boccardo F, Guglielmini P, Parodi A, Rubagotti A. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, oestrogen receptor-positive breast cancer patients: very late results of the 'gruppo di ricerca per la chemio-ormonoterapia adiuvante (GROCTA)' 01-Trial in early breast cancer. Breast Cancer Res Treat. 2011; 126:653–61.
Article6. Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M, et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002; 20:4628–35.
Article7. von Minckwitz G, Graf E, Geberth M, Eiermann W, Jonat W, Conrad B, et al. CMF versus goserelin as adjuvant therapy for node-negative, hormone-receptor-positive breast cancer in premenopausal patients: a randomised trial (GABG trial IV-A-93). Eur J Cancer. 2006; 42:1780–8.
Article8. Bernhard J, Zahrieh D, Castiglione-Gertsch M, Hurny C, Gelber RD, Forbes JF, et al. Adjuvant chemotherapy followed by goserelin compared with either modality alone: the impact on amenorrhea, hot flashes, and quality of life in premenopausal patients--the International Breast Cancer Study Group Trial VIII. J Clin Oncol. 2007; 25:263–70.
Article9. de Haes H, Olschewski M, Kaufmann M, Schumacher M, Jonat W, Sauerbrei W, et al. Quality of life in goserelin-treated versus cyclophosphamide + methotrexate + fluorouracil-treated premenopausal and perimenopausal patients with node-positive, early breast cancer: the Zoladex Early Breast Cancer Research Association Trialists Group. J Clin Oncol. 2003; 21:4510–6.
Article10. Nystedt M, Berglund G, Bolund C, Fornander T, Rutqvist LE. Side effects of adjuvant endocrine treatment in premenopausal breast cancer patients: a prospective randomized study. J Clin Oncol. 2003; 21:1836–44.
Article11. Hurny C, Bernhard J, Coates AS, Castiglione-Gertsch M, Peterson HF, Gelber RD, et al. Impact of adjuvant therapy on quality of life in women with node-positive operable breast cancer. International Breast Cancer Study Group. Lancet. 1996; 347:1279–84.12. Bernhard J, Zahrieh D, Coates AS, Gelber RD, Castiglione-Gertsch M, Murray E, et al. Quantifying trade-offs: quality of life and quality-adjusted survival in a randomised trial of chemotherapy in postmenopausal patients with lymph node-negative breast cancer. Br J Cancer. 2004; 91:1893–901.
Article13. Roche H, Kerbrat P, Bonneterre J, Fargeot P, Fumoleau P, Monnier A, et al. Complete hormonal blockade versus epirubicin-based chemotherapy in premenopausal, one to three node-positive, and hormone-receptor positive, early breast cancer patients: 7-year follow-up results of French Adjuvant Study Group 06 randomised trial. Ann Oncol. 2006; 17:1221–7.14. Kim HJ, Lee JS, Park EH, Lim WS, Sei JY, Koh BS, et al. Short term results from GHRH analogue use in pre-menopausal breast cancer in Korea. Eur J Surg Oncol. 2009; 35:936–41.
Article15. Kaufmann M, Jonat W, Blamey R, Cuzick J, Namer M, Fogelman I, et al. Survival analyses from the ZEBRA study: goserelin (Zoladex) versus CMF in premenopausal women with node-positive breast cancer. Eur J Cancer. 2003; 39:1711–7.16. International Breast Cancer Study Group (IBCSG), Castiglione-Gertsch M, O'Neill A, Price KN, Goldhirsch A, Coates AS, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial. J Natl Cancer Inst. 2003; 95:1833–46.17. Shiba E, Yamashita H, Kurebayashi J, Noguchi S, Iwase H, Ohashi Y, et al. A randomized controlled study evaluating safety and efficacy of leuprorelin acetate every-3-months depot for 2 versus 3 or more years with tamoxifen for 5 years as adjuvant treatment in premenopausal patients with endocrine-responsive breast cancer. Breast Cancer. 2016; 23:499–509.18. Boccardo F, Rubagotti A, Amoroso D, Mesiti M, Romeo D, Sismondi P, et al. Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-/perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group 02 randomized trial. J Clin Oncol. 2000; 18:2718–27.
Article19. Jakesz R, Hausmaninger H, Kubista E, Gnant M, Menzel C, Bauernhofer T, et al. Randomized adjuvant trial of tamoxifen and goserelin versus cyclophosphamide, methotrexate, and fluorouracil: evidence for the superiority of treatment with endocrine blockade in premenopausal patients with hormone-responsive breast cancer: Austrian Breast and Colorectal Cancer Study Group Trial 5. J Clin Oncol. 2002; 20:4621–7.20. Roche H, Mihura J, de Lafonta B, Reme-Saumon M, Martel P, Dubois J, et al. Castration and tamoxifen versus chemotherapy (FAC) for premenopausal, node negative, recpetor positive breast cancer patients: a randomized trail with a 7 years median follow-up. Proc Am Soc Clin Oncol. 1996; 15:117.21. Swain SM, Jeong JH, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010; 362:2053–65.
Article22. Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 365:1687–717.23. LHRH-agonists in Early Breast Cancer Overview Group, Cuzick J, Ambroisine L, Davidson N, Jakesz R, Kaufmann M, et al. Use of luteinising-hormone-releasing hormone agonists as adjuvant treatment in premenopausal patients with hormone-receptor-positive breast cancer: a meta-analysis of individual patient data from randomised adjuvant trials. Lancet. 2007; 369:1711–23.24. Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients. The International Breast Cancer Study Group. Ann Oncol. 1990; 1:183–8.25. Bianco AR, Del Mastro L, Gallo C, Perrone F, Matano E, Pagliarulo C, et al. Prognostic role of amenorrhea induced by adjuvant chemotherapy in premenopausal patients with early breast cancer. Br J Cancer. 1991; 63:799–803.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Can chemotherapy be omitted for patients with N0 or N1 endocrine-sensitive breast cancer treated with gonadotropin-releasing hormone agonist and tamoxifen?
- Role of Ovarian Function Suppression in Premenopausal Women with Early Breast Cancer
- Comparison of Tamoxifen and Toremifene as Adjuvant Treatment in Node-negative Postmenopausal Breast Cancer
- Radiation recall dermatitis induced by tamoxifen during adjuvant breast cancer treatment
- Personalized therapy for advanced breast cancer using molecular signatures