Nat Prod Sci.
2016 Sep;22(3):175-179. 10.20307/nps.2016.22.3.175.
Beneficial Effect of Lespedeza cuneata (G. Don) Water Extract on Streptozotocin-induced Type 1 Diabetes and Cytokine-induced Beta-cell Damage
- Affiliations
-
- 1Department of Oriental Medicine Resources and Institute of Korean Medicine Industry, Mokpo National University, Jeonnam 534-729, Republic of Korea. rhyudy@mokpo.ac.kr
- KMID: 2355142
- DOI: http://doi.org/10.20307/nps.2016.22.3.175
Abstract
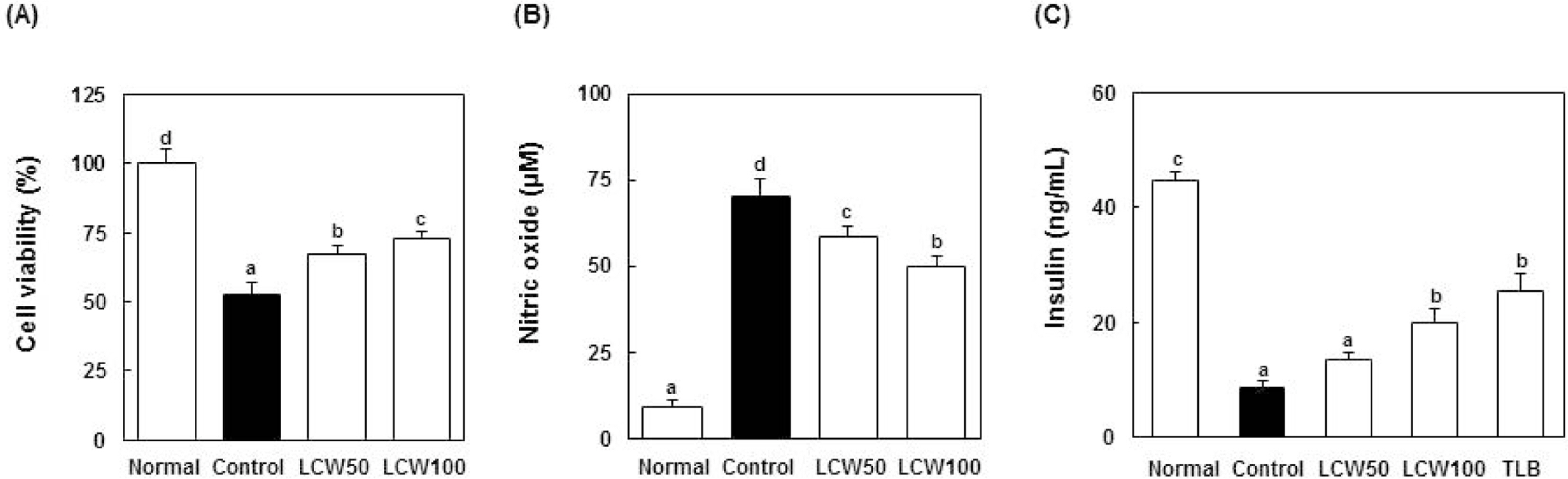
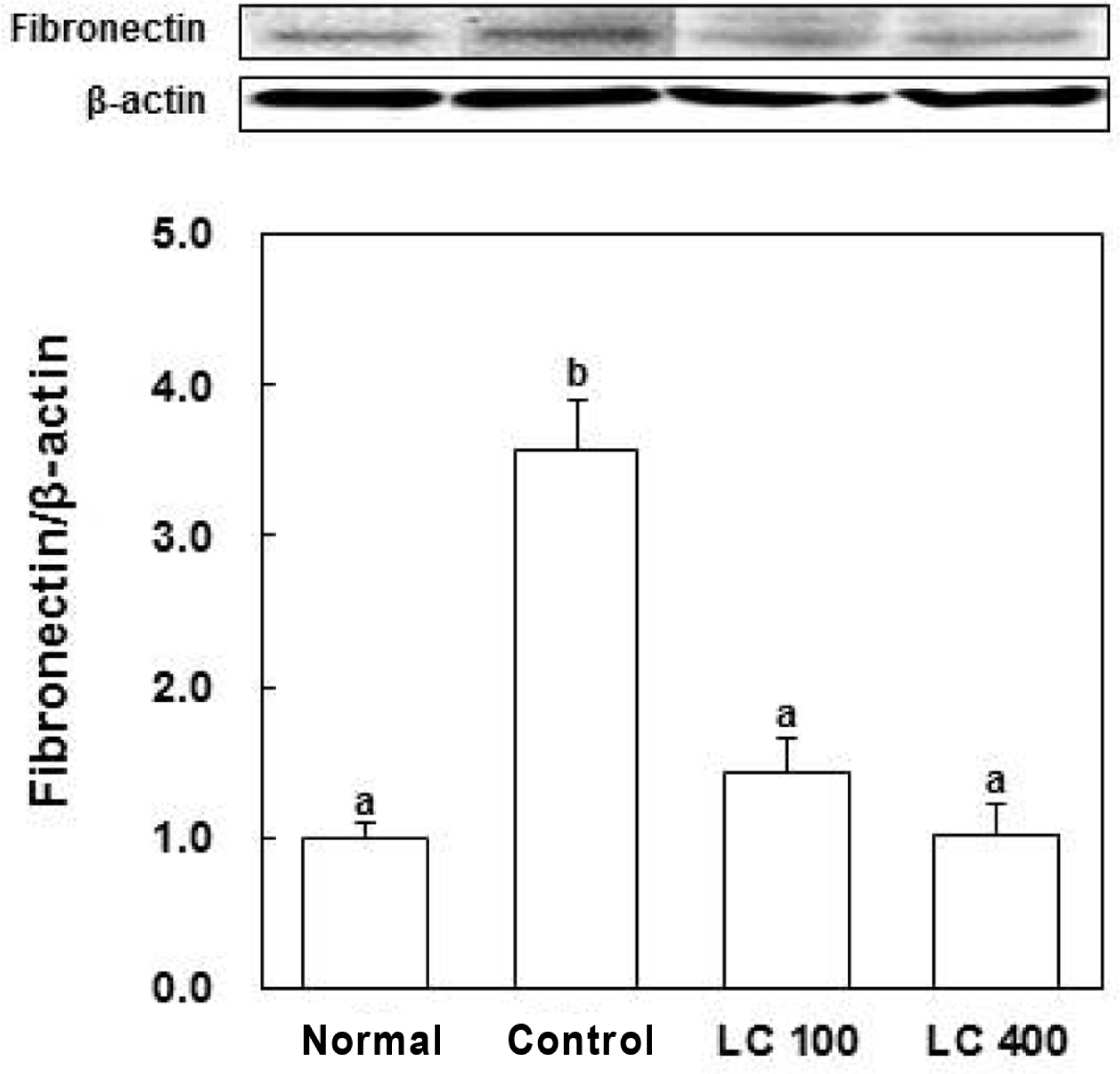
- The aim of this study was to evaluate the anti-diabetic effects of the water extract of Lespedeza cuneata (LCW) using rat insulinoma (RIN) m5F cells and streptozotocin (STZ)-induced diabetic rats. The effect of LCW on the protection of pancreatic beta cells was assessed using MTT assay, and nitric oxide production was assessed using Griess reagent. STZ-induced diabetic rats were treated with 100 and 400 mg/kg body weight of LCW for 5 weeks. In results, LCW significantly protected cytokine-induced toxicity and NO production, and increased insulin secretion in RINm5F cells. LCW significantly decreased serum blood glucose, thiobarbituric acid reactive substances (TBARS), blood urea nitrogen (BUN) and advanced glycation end products (AGEs) levels, and renal fibronectin expression in STZ-induced diabetic rats. Also, LCW effectively improved BW loss in STZ-induced diabetic rats. Thus, our results suggest that LCW has a beneficial effect on cytokine-induced pancreatic beta cell damage and biomarkers of diabetic complication in hyperglycemic rats.
MeSH Terms
-
Animals
Biomarkers
Blood Glucose
Blood Urea Nitrogen
Body Weight
Cytokines
Diabetes Complications
Fibronectins
Glycosylation End Products, Advanced
Insulin
Insulin-Secreting Cells
Insulinoma
Lespedeza*
Nitric Oxide
Rats
Streptozocin
Thiobarbituric Acid Reactive Substances
Water*
Biomarkers
Blood Glucose
Cytokines
Fibronectins
Glycosylation End Products, Advanced
Insulin
Nitric Oxide
Streptozocin
Thiobarbituric Acid Reactive Substances
Water
Figure
Reference
-
(1). Yoon J. W., Jun H. S. Ann. N. Y.Acad. Sci. 2001; 928:200–211.(2). Sharma B. R., Rhyu D. Y. Asian Pac. J.Trop. Biomed. 2014; 4:575–580.(3). Lenzen S.Diabetologia. 2008; 51:216–226.(4). Prabhakar P. K., Doble M. Chin. J.Integr. Med. 2011; 17:563–574.(5). Deng F., Chang J., Zhang J. S. J.Asian Nat. Prod. Res. 2007; 9:655–658.(6). Huang K. C., Williams W. M.The Pharmacology of Chinese Herbs. CRC Press;USA: 1999.(7). Sharma B. R., Kim M. S., Takako Y., Rhyu D. Y. J.Med. Plant Res. 2014; 8:935–941.(8). Sen S., De B., Devanna N., Chakraborty R. Chin. J.Nat. Med. 2013; 11:149–157.(9). Sharma B. R., Rhyu D. Y.BioMed. Res. Int. 2015; 169256.(10). Punithavathi V. R., Prince P. S., Kumar R., Selvakumari J.Eur. J. Pharmacol. 2011; 650:465–471.(11). Stanley M. P. P., Kamalakkannan N. J.Biochem. Mol. Toxicol. 2006; 20:96–102.
Article(12). Song M. Y., Bae U. J., Lee B. H., Kwon K. B., Seo E. A., Park S. J., Kim M. S., Song H. J., Kwon K. S., Park J. W., Ryu D. G., Park B. H.World J. Gastroenterol. 2010; 16:3249–3257.(13). Hafizur R. M., Kabir N., Chishti S. Br. J.Nutr. 2012; 108:1586–1595.(14). Dabla P. K.World J. Diabetes. 2010; 1:48–56. 2012.(15). Lin N., Zhang H., Su Q.Diabetes Metab. 2012; 38:250–257.(16). Gawel S., Wardas M., Niedworok E., Wardas P.Wiad. Lek. 2004; 57:453–455.(17). Xin X., Khan Z. A., Chen S., Chakrabarti S.Lab. Invest. 2004; 84:1451–1459.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced beta-cell damage and the development of type 1 diabetes
- Sulfuretin protects against cytokine-induced beta-cell damage and prevents streptozotocin-induced diabetes
- Therapeutic Effect of Amomum xanthoides Extract on Experimental Diabetes Induced by Alloxan
- Effect of the magnetized water supplementation on blood glucose, lymphocyte DNA damage, antioxidant status, and lipid profiles in STZ-induced rats
- Effects of Benincasa hispida Fractions on Hepatic Lipid Levels and Lipid Peroxidation in Streptozotocin Induced Diabetic Rats