J Clin Neurol.
2016 Apr;12(2):188-193. 10.3988/jcn.2016.12.2.188.
Cerebral Cortex Involvement in Neuromyelitis Optica Spectrum Disorder
- Affiliations
-
- 1Department of Neurology, The Catholic University of Korea College of Medicine, Seoul, Korea. wjkim@catholic.ac.kr
- 2Department of Neurology, Research Institute and Hospital of National Cancer Center, Goyang, Korea.
- 3Department of Neurology, Kosin University College of Medicine, Busan, Korea.
- 4Department of Neurology, Yeungnam University College of Medicine, Daegu, Korea.
- 5Department of Neurology, Inje University Ilsan Paik Hospital, Goyang, Korea.
- 6Department of Radiolgoy, Research Institute and Hospital of National Cancer Center, Goyang, Korea.
- KMID: 2354139
- DOI: http://doi.org/10.3988/jcn.2016.12.2.188
Abstract
- BACKGROUND AND PURPOSE
Brain lesions involving the cerebral cortex are rarely described in patients with neuromyelitis optica spectrum disorder (NMOSD), in contrast to multiple sclerosis. We investigated cerebral cortex involvement using conventional brain magnetic resonance imaging (MRI) in anti-aquaporin-4 (AQP4)-antibody-positive NMOSD patients.
METHODS
The study enrolled 215 NMOSD patients who were seropositive for the anti-AQP4 antibody from 5 referral hospitals, and retrospectively analyzed their demographic, clinical, and MRI findings. Abnormal cerebral cortex lesions on brain MRI were identified by a neuroradiologist and two neurologists using consensus.
RESULTS
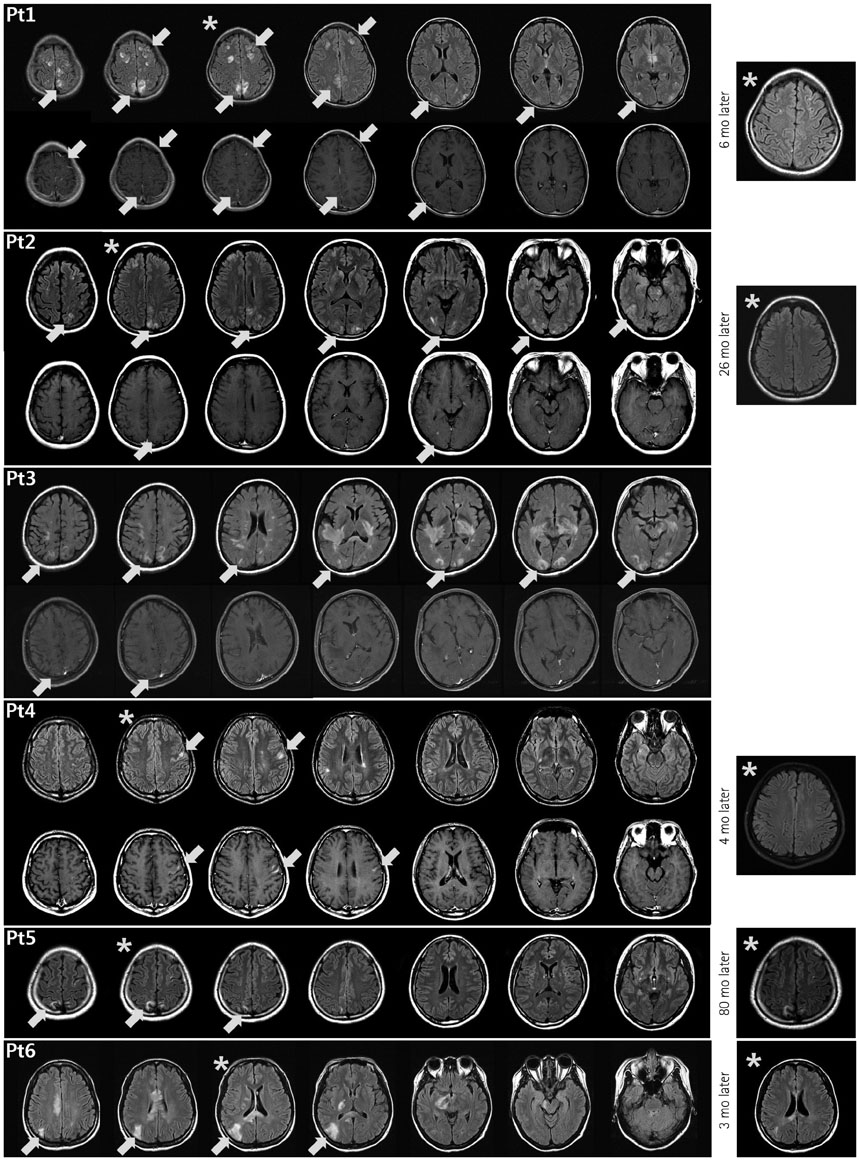
Most of the 215 enrolled patients (87%) were female. The median age at onset was 22.5 years (range: 15-36 years) and the mean follow-up duration was 123 months. Brain lesions were found in 143 of 194 patients (74%) in whom MRI was performed during follow-up. Brain lesions involving the cerebral cortex were identified in 6 of these 194 patients (3.1%). Five of the patients were female, and the six patients together had a median age of 29 years (range: 15-36 years) at the time of lesion presentation. Three of them showed leptomeningeal enhancement in the lesions. At presentation of the cortex-involving lesions, five of these patients were not being treated at the time of presentation, while the sixth was being treated with interferon-beta.
CONCLUSIONS
Although rare, cortical involvement occurs in NMOSD and is commonly combined with leptomeningeal enhancement. We speculate that this occurs only in patients who are not treated appropriately with immunosuppressant drugs.
Keyword
MeSH Terms
Figure
Reference
-
1. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015; 85:177–189.
Article2. Kim W, Kim SH, Kim HJ. New insights into neuromyelitis optica. J Clin Neurol. 2011; 7:115–127.
Article3. Kim HJ, Paul F, Lana-Peixoto MA, Tenembaum S, Asgari N, Palace J, et al. MRI characteristics of neuromyelitis optica spectrum disorder: an international update. Neurology. 2015; 84:1165–1173.
Article4. Kim JE, Kim SM, Ahn SW, Lim BC, Chae JH, Hong YH, et al. Brain abnormalities in neuromyelitis optica. J Neurol Sci. 2011; 302:43–48.
Article5. Kim SM, Kim JS, Heo YE, Yang HR, Park KS. Cortical oscillopsia without nystagmus, an isolated symptom of neuromyelitis optica spectrum disorder with anti-aquaporin 4 antibody. Mult Scler. 2012; 18:244–247.
Article6. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007; 6:805–815.
Article7. Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004; 364:2106–2112.
Article8. Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med. 2006; 210:307–313.
Article9. Kim W, Lee JE, Li XF, Kim SH, Han BG, Lee BI, et al. Quantitative measurement of anti-aquaporin-4 antibodies by enzyme-linked immunosorbent assay using purified recombinant human aquaporin-4. Mult Scler. 2012; 18:578–586.
Article10. Calabrese M, Oh MS, Favaretto A, Rinaldi F, Poretto V, Alessio S, et al. No MRI evidence of cortical lesions in neuromyelitis optica. Neurology. 2012; 79:1671–1676.
Article11. Kister I, Herbert J, Zhou Y, Ge Y. Ultrahigh-field MR (7 T) imaging of brain lesions in neuromyelitis optica. Mult Scler Int. 2013; 2013:398259.12. Popescu BF, Parisi JE, Cabrera-Gómez JA, Newell K, Mandler RN, Pittock SJ, et al. Absence of cortical demyelination in neuromyelitis optica. Neurology. 2010; 75:2103–2109.
Article13. Cabrera-Gomez JA, Kister I. Conventional brain MRI in neuromyelitis optica. Eur J Neurol. 2012; 19:812–819.
Article14. Puthenparampil M, Poggiali D, Causin F, Rolma G, Rinaldi F, Perini P, et al. Cortical relapses in multiple sclerosis. Mult Scle. 2015; [Epub ahead of print].
Article15. Kim W, Kim SH, Lee SH, Li XF, Kim HJ. Brain abnormalities as an initial manifestation of neuromyelitis optica spectrum disorder. Mult Scler. 2011; 17:1107–1112.
Article16. Kim SH, Huh SY, Hyun JW, Jeong IH, Lee SH, Joung A, et al. A longitudinal brain magnetic resonance imaging study of neuromyelitis optica spectrum disorder. PLoS One. 2014; 9:e108320.
Article17. Wingerchuk DM, Weinshenker BG. Acute disseminated encephalomyelitis, transverse myelitis, and neuromyelitis optica. Continuum (Minneap Minn). 2013; 19(4 Multiple Sclerosis):944–967.
Article18. Okumura A, Nakazawa M, Igarashi A, Abe S, Ikeno M, Nakahara E, et al. Anti-aquaporin 4 antibody-positive acute disseminated encephalomyelitis. Brain Dev. 2015; 37:339–343.
Article19. Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009; 72:712–717.
Article20. Long Y, Chen M, Zhang B, Gao C, Zheng Y, Xie L, et al. Brain gadolinium enhancement along the ventricular and leptomeningeal regions in patients with aquaporin-4 antibodies in cerebral spinal fluid. J Neuroimmunol. 2014; 269:62–67.
Article21. Pittock SJ, Lennon VA, de Seze J, Vermersch P, Homburger HA, Wingerchuk DM, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol. 2008; 65:78–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postpartum Relapse of Neuromyelitis Optica Spectrum Disorder after a Long Period of Spontaneous Remission
- Isolated Tongue Paralysis as Presentation of Seropositive Neuromyelitis Optica Spectrum Disorder
- Early relapse after rituximab treatment in a patient with seronegative neuromyelitis optica spectrum disorder: a case report
- Narcolepsy Followed by Intractable Vomiting Caused by Recurrent Brain Involvement in Neuromyelitis Optica Spectrum Disorder
- A Case of Cerebral Salt Wasting Syndrome in Neuromyelitis Optica Spectrum Disorder