Endocrinol Metab.
2016 Sep;31(3):462-468. 10.3803/EnM.2016.31.3.462.
Comparison of Thyroglobulin Measurements Using Three Different Immunoassay Kits: A BRAMHS Tg-Plus RIA Kit, a BRAMHS hTg Sensitive Kryptor Kit, and a Beckman Coulter ACCESS Immunoassay Kit
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. mj080332@gmail.com
- 2Department of Nuclear Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2352987
- DOI: http://doi.org/10.3803/EnM.2016.31.3.462
Abstract
- BACKGROUND
Second-generation thyroglobulin immunometric assays (Tg-IMAs) have been developed with improved sensitivity. Our aim was to compare the diagnostic value of Tg-IMA measurements using a Kryptor (BRAHMS AG) kit (Tg-K) and an ACCESS (Beckman Coulter) kit (Tg-A) with that of the first-generation Tg measurement using a Tg-plus (BRAHMS AG) kit (Tg+).
METHODS
We enrolled 82 differentiated thyroid cancer patients who underwent total thyroidectomy with radioactive iodine remnant ablation and who underwent diagnostic whole body scan using recombinant human thyroid stimulating hormone (rhTSH). The Tg+, Tg-K, and Tg-A were measured before rhTSH administration during levothyroxine treatment (suppressed Tg) from the same sample. Serum Tg+ was measured after rhTSH stimulation (stimulated Tg).
RESULTS
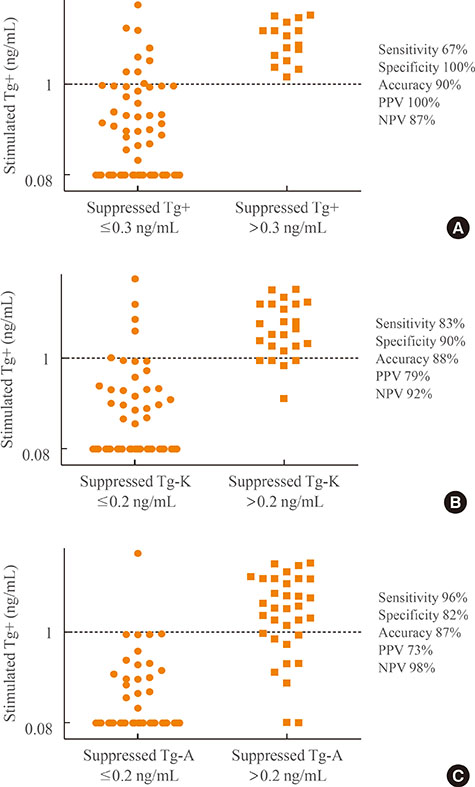
Suppressed Tg+ was more significantly correlated with suppressed Tg-K (R²=0.919, P<0.001) than with suppressed Tg-A (R²=0.536, P<0.001). The optimal cut-off values of suppressed Tg+, Tg-K, and Tg-A for predicting stimulated Tg+ of 1 ng/mL were 0.3, 0.2, and 0.2 ng/mL, respectively. The sensitivity, specificity, and accuracy of suppressed Tg+ were 67%, 100%, and 90%, respectively; those of suppressed Tg-K were 83%, 90%, and 88%; those of suppressed Tg-A were 96%, 82%, and 87%, respectively. The positive predictive and negative predictive values of Tg+ were 100% and 87%, respectively; those of Tg-K were 79% and 92%; and those of Tg-A were 73% and 98%.
CONCLUSION
We could not clearly demonstrate which kit had better diagnostic performance after comparison of first-generation Tg measurements with Tg-IMA measurements. Also, there were kit-to-kit variations between Tg-IMA kits. Suppressed Tg measured by Tg-IMA was insufficient to completely substitute for a stimulated Tg measurement.
MeSH Terms
Figure
Reference
-
1. Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 see comments. Cancer. 1998; 83:2638–2648.2. Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, et al. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010; 20:1341–1349.3. McGrath RT, Preda VA, Clifton-Bligh P, Robinson B, Sywak M, Delbridge L, et al. Is there a role for an ultrasensitive thyroglobulin assay in patients with serum antithyroglobulin antibodies? A large (Australian) cohort study in differentiated thyroid cancer. Clin Endocrinol (Oxf). 2015; 02. 05. [Epub]. DOI: 10.1111/cen.12736.4. Spencer C, Petrovic I, Fatemi S, LoPresti J. Serum thyroglobulin (Tg) monitoring of patients with differentiated thyroid cancer using sensitive (second-generation) immunometric assays can be disrupted by false-negative and false-positive serum thyroglobulin autoantibody misclassifications. J Clin Endocrinol Metab. 2014; 99:4589–4599.5. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009; 19:1167–1214.6. Smallridge RC, Meek SE, Morgan MA, Gates GS, Fox TP, Grebe S, et al. Monitoring thyroglobulin in a sensitive immunoassay has comparable sensitivity to recombinant human TSH-stimulated thyroglobulin in follow-up of thyroid cancer patients. J Clin Endocrinol Metab. 2007; 92:82–87.7. Castagna MG, Brilli L, Pilli T, Montanaro A, Cipri C, Fioravanti C, et al. Limited value of repeat recombinant human thyrotropin (rhTSH)-stimulated thyroglobulin testing in differentiated thyroid carcinoma patients with previous negative rhTSH-stimulated thyroglobulin and undetectable basal serum thyroglobulin levels. J Clin Endocrinol Metab. 2008; 93:76–81.8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016; 26:1–133.9. Schlumberger M, Pacini F, Wiersinga WM, Toft A, Smit JW, Sanchez Franco F, et al. Follow-up and management of differentiated thyroid carcinoma: a European perspective in clinical practice. Eur J Endocrinol. 2004; 151:539–548.10. Moon JH, Choi JY, Jeong WJ, Ahn SH, Lee WW, Kim KM, et al. Recombinant human thyrotropin-stimulated thyroglobulin level at the time of radioactiveiodine ablation is an independent prognostic marker of differentiated thyroid carcinoma in the setting of prophylactic central neck dissection. Clin Endocrinol (Oxf). 2016; 85:459–465.11. Evans C, Tennant S, Perros P. Thyroglobulin in differentiated thyroid cancer. Clin Chim Acta. 2015; 444:310–317.12. Wunderlich G, Zophel K, Crook L, Smith S, Smith BR, Franke WG. A high-sensitivity enzyme-linked immunosorbent assay for serum thyroglobulin. Thyroid. 2001; 11:819–824.13. Iervasi A, Iervasi G, Bottoni A, Boni G, Annicchiarico C, Di Cecco P, et al. Diagnostic performance of a new highly sensitive thyroglobulin immunoassay. J Endocrinol. 2004; 182:287–294.14. Zophel K, Wunderlich G, Smith BR. Serum thyroglobulin measurements with a high sensitivity enzyme-linked immunosorbent assay: is there a clinical benefit in patients with differentiated thyroid carcinoma? Thyroid. 2003; 13:861–865.15. Iervasi A, Iervasi G, Ferdeghini M, Solimeo C, Bottoni A, Rossi L, et al. Clinical relevance of highly sensitive Tg assay in monitoring patients treated for differentiated thyroid cancer. Clin Endocrinol (Oxf). 2007; 67:434–441.16. Schlumberger M, Hitzel A, Toubert ME, Corone C, Troalen F, Schlageter MH, et al. Comparison of seven serum thyroglobulin assays in the follow-up of papillary and follicular thyroid cancer patients. J Clin Endocrinol Metab. 2007; 92:2487–2495.17. Spencer CA, Bergoglio LM, Kazarosyan M, Fatemi S, LoPresti JS. Clinical impact of thyroglobulin (Tg) and Tg autoantibody method differences on the management of patients with differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2005; 90:5566–5575.18. Ross HA, Netea-Maier RT, Schakenraad E, Bravenboer B, Hermus AR, Sweep FC. Assay bias may invalidate decision limits and affect comparability of serum thyroglobulin assay methods: an approach to reduce interpretation differences. Clin Chim Acta. 2008; 394:104–109.19. Giovanella L, Ceriani L, Ghelfo A, Maffioli M, Keller F. Preoperative undetectable serum thyroglobulin in differentiated thyroid carcinoma: incidence, causes and management strategy. Clin Endocrinol (Oxf). 2007; 67:547–551.20. Spencer CA, Takeuchi M, Kazarosyan M. Current status and performance goals for serum thyroglobulin assays. Clin Chem. 1996; 42:164–173.21. Spencer CA, Lopresti JS. Measuring thyroglobulin and thyroglobulin autoantibody in patients with differentiated thyroid cancer. Nat Clin Pract Endocrinol Metab. 2008; 4:223–233.22. Giovanella L. Highly sensitive thyroglobulin measurements in differentiated thyroid carcinoma management. Clin Chem Lab Med. 2008; 46:1067–1073.23. Giovanella L, Clark PM, Chiovato L, Duntas L, Elisei R, Feldt-Rasmussen U, et al. Thyroglobulin measurement using highly sensitive assays in patients with differentiated thyroid cancer: a clinical position paper. Eur J Endocrinol. 2014; 171:R33–R46.24. Spencer C, LoPresti J, Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes. 2014; 21:394–404.25. Giovanella L, Treglia G, Sadeghi R, Trimboli P, Ceriani L, Verburg FA. Unstimulated highly sensitive thyroglobulin in follow-up of differentiated thyroid cancer patients: a meta-analysis. J Clin Endocrinol Metab. 2014; 99:440–447.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of the Precision and Functional Sensitivity of Two Thyroglobulin Assays: Comparison of the Second-Generation Roche Electrochemiluminescent Immunoassay and BRAHAMS Radioimmunoassay
- Effects of Anti-thyroglobulin Antibody on the Measurement of Thyroglobulin: Differences Between Immunoradiometric Assay Kits Available
- Clinical evaluation of a rapid diagnostic test kit for detection of canine coronavirus
- Evaluation of the VIDAS CDAB Kits for the Detection of the Clostridium difficile Toxins A and B
- Performance Evaluation of the Serum Thyroglobulin Assays With Immunochemiluminometric Assay and Immunoradiometric Assay for Differentiated Thyroid Cancer