Transl Clin Pharmacol.
2016 Sep;24(3):127-131. 10.12793/tcp.2016.24.3.127.
Rooibosâ„¢: Automated schedule broadcast software for clinical pharmacology studies
- Affiliations
-
- 1Clinical Pharmacology Unit, Biomedical Research Institute of Chonbuk National University Hospital, Jeonju 54907, Republic of Korea. mgkim@jbnu.ac.kr
- 2CAMTIC Advanced Mechatronics Technology Institute for Commercialization, Jeonju 54852, Republic of Korea.
- 3Department of Urology, Chonbuk National University Hospital, Jeonju 54907, Republic of Korea.
- 4Division of Endocrinology and Metabolism, Department of Internal Medicine, Chonbuk National University Hospital, Jeonju 54907, Republic of Korea.
- 5Department of Pharmacology, Chonbuk National University Medical School, Jeonju 54907, Republic of Korea.
- KMID: 2351798
- DOI: http://doi.org/10.12793/tcp.2016.24.3.127
Abstract
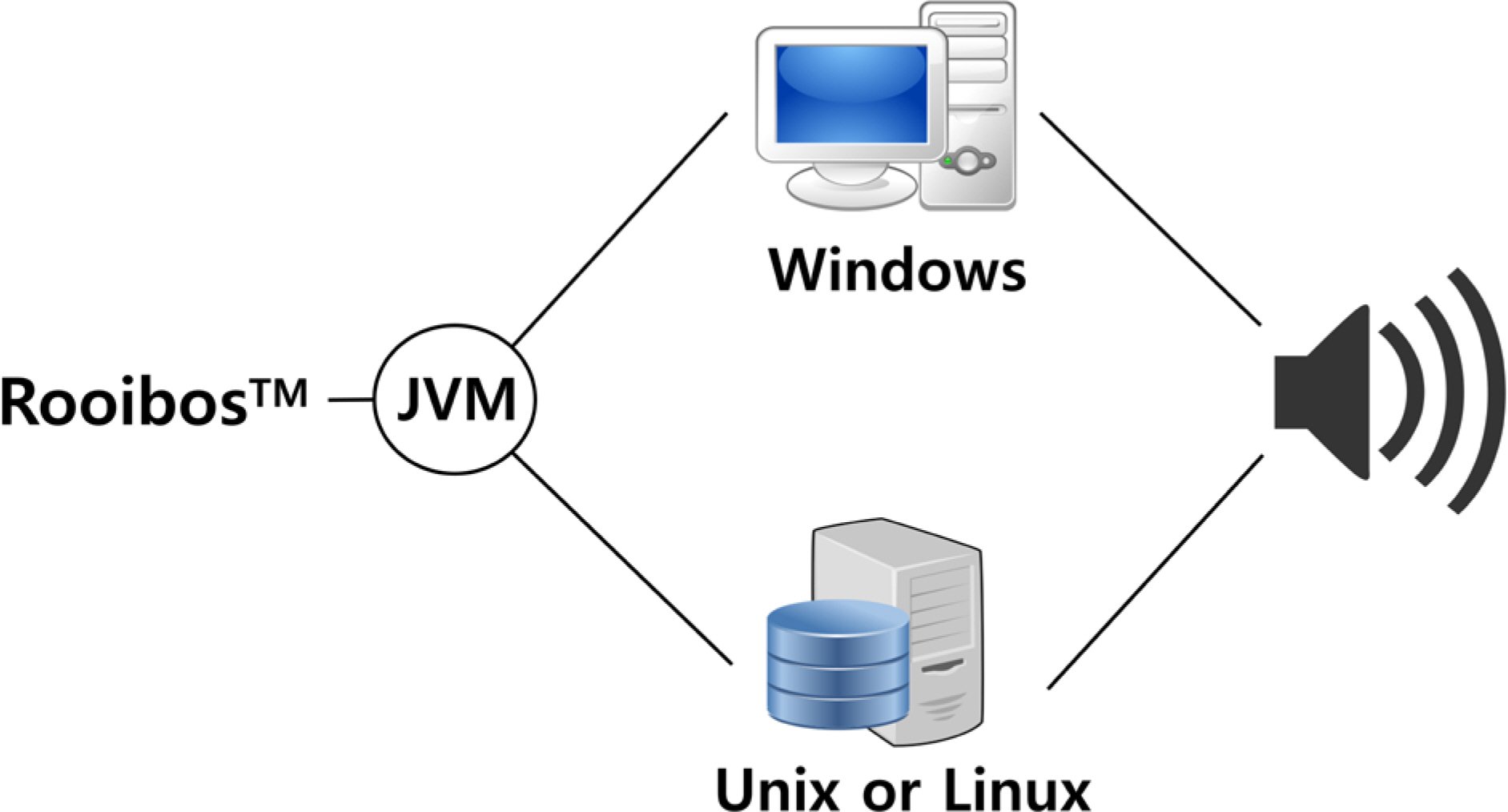
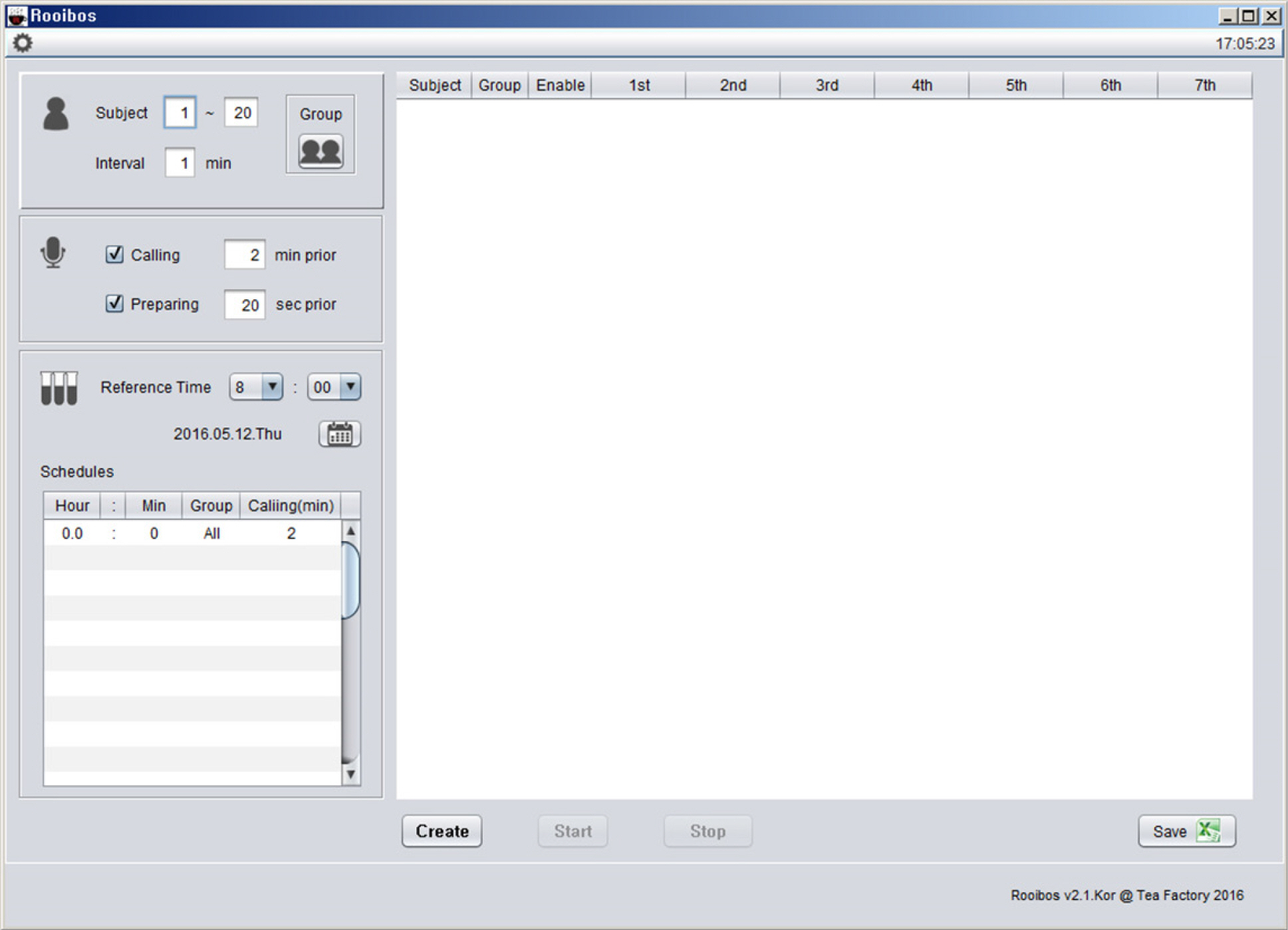
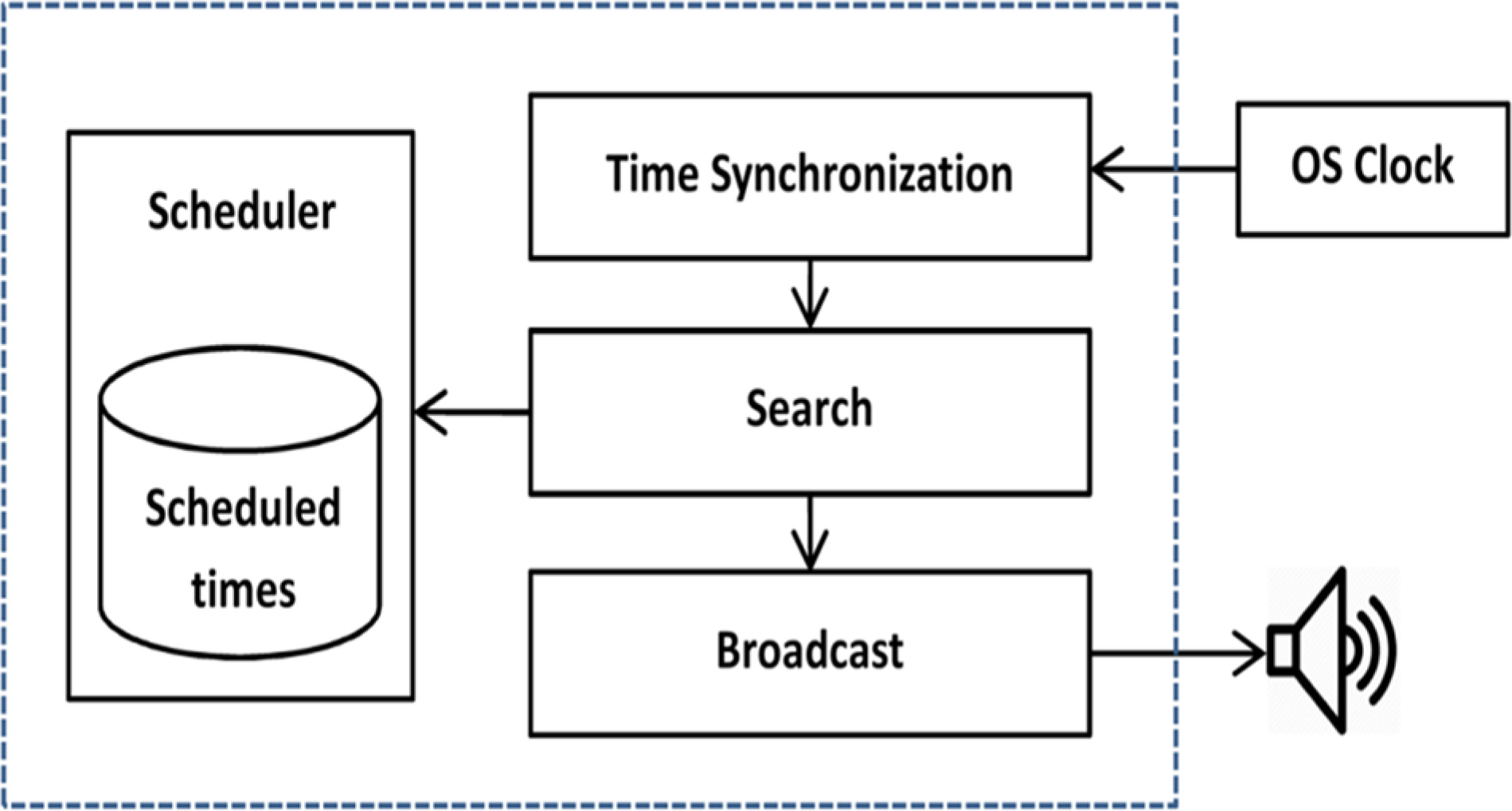
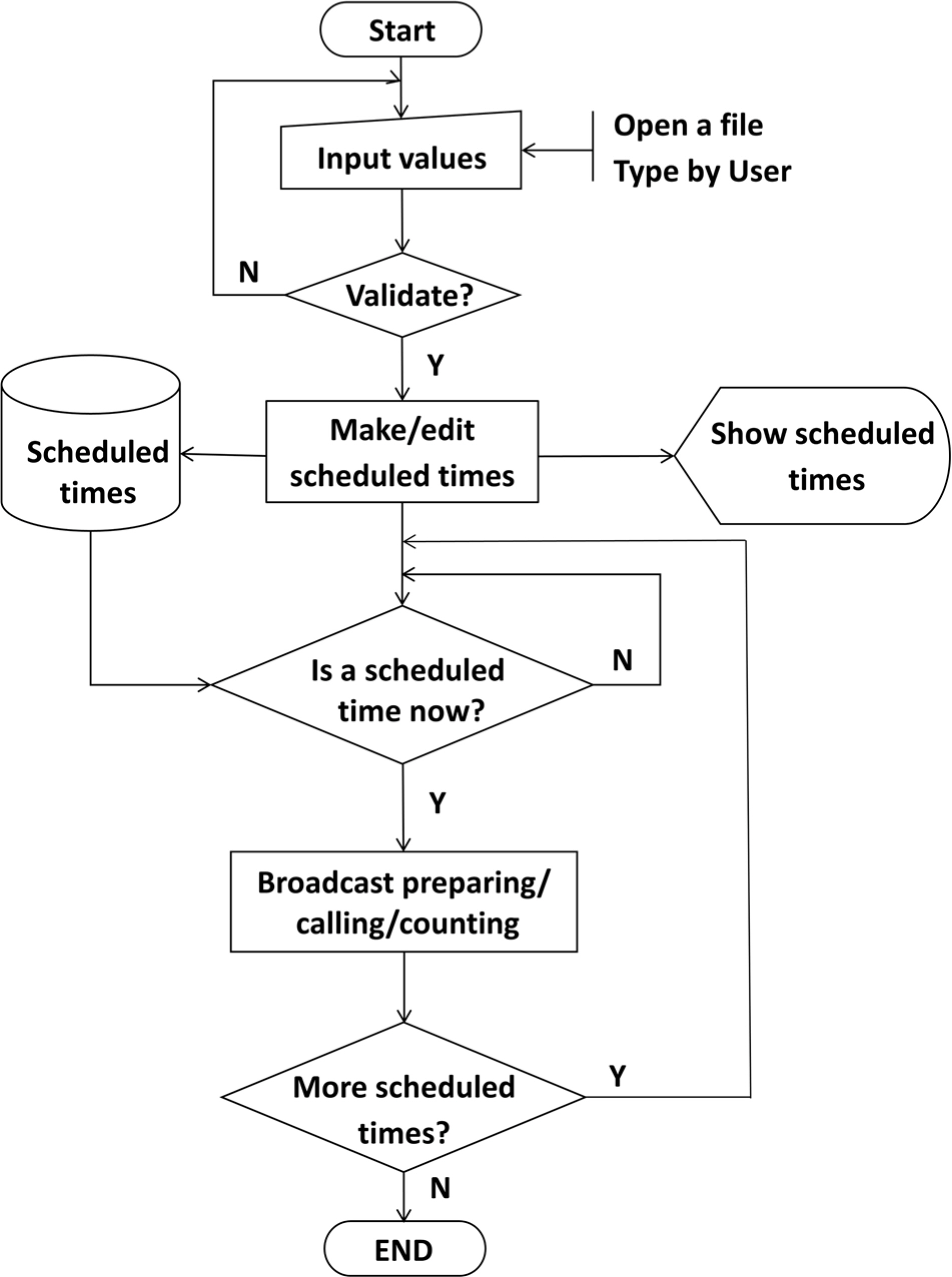
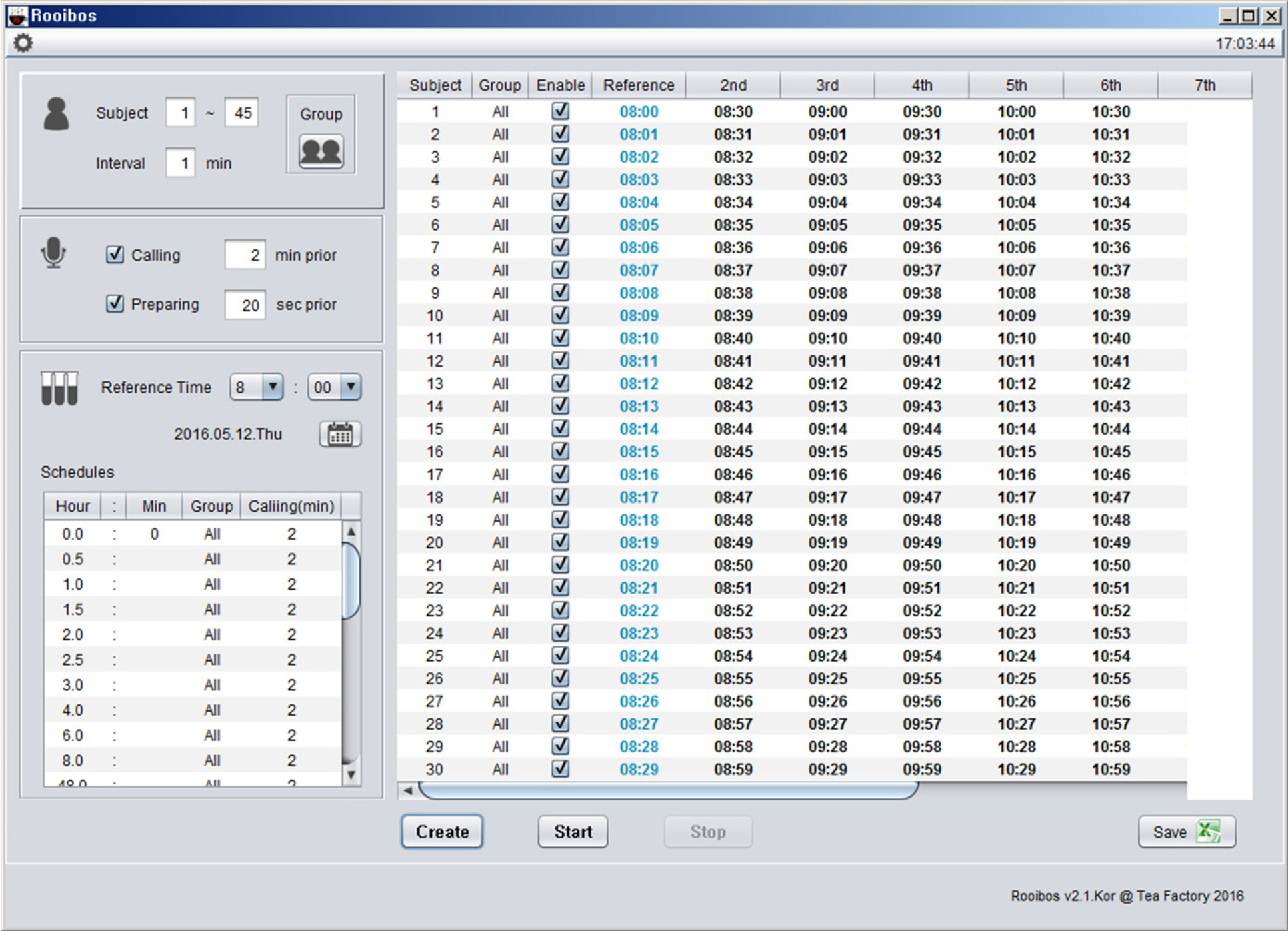
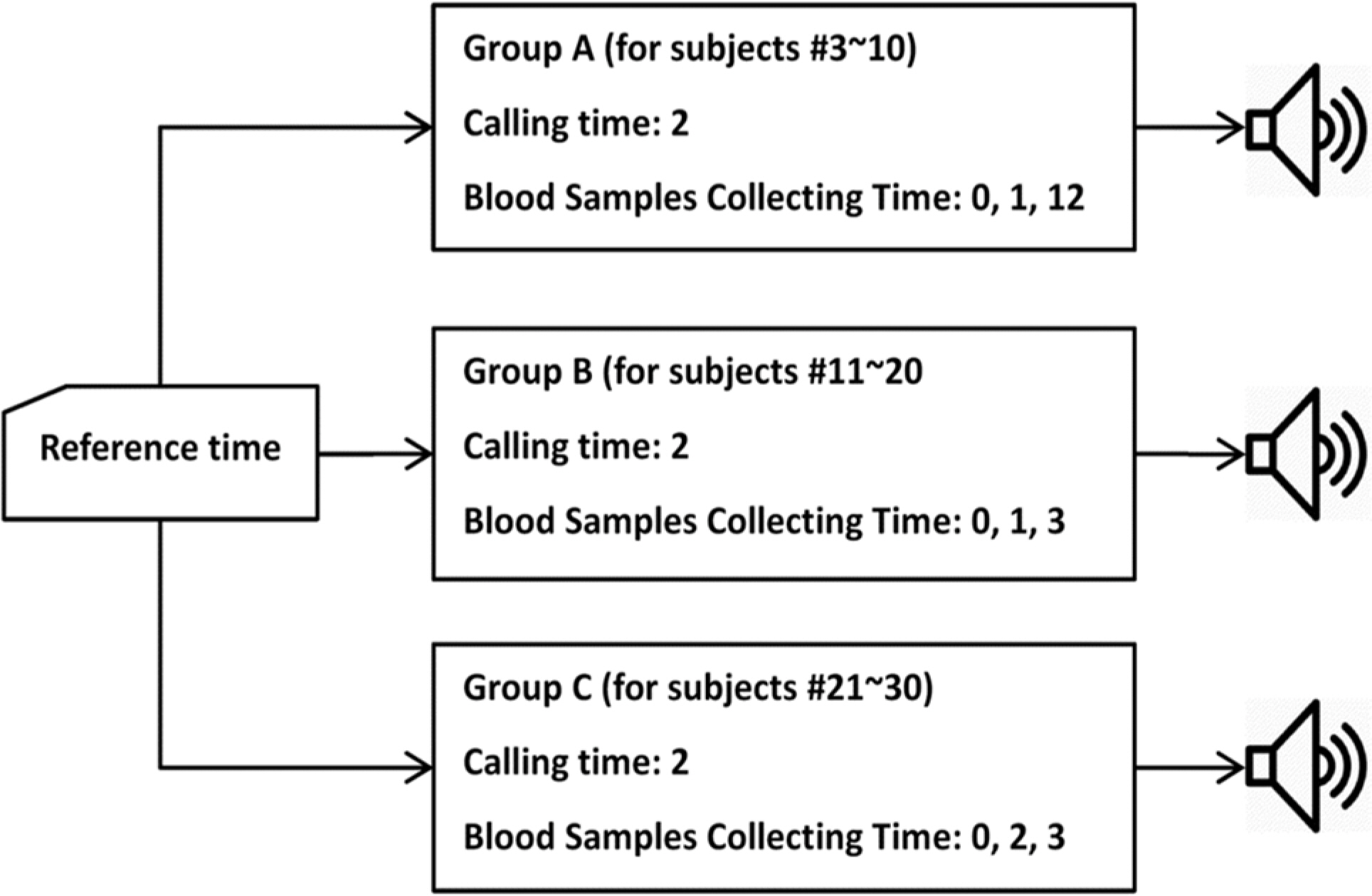
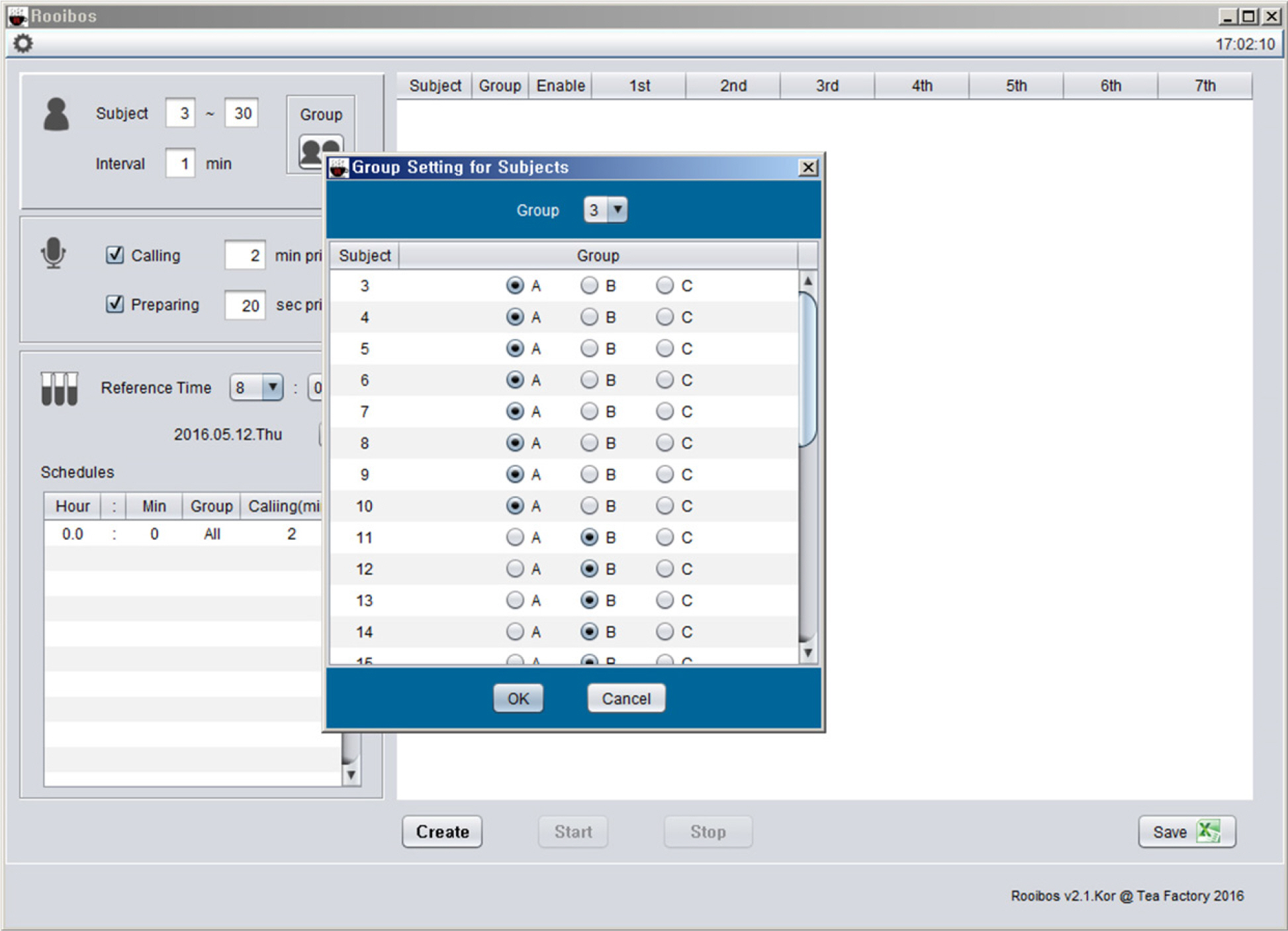
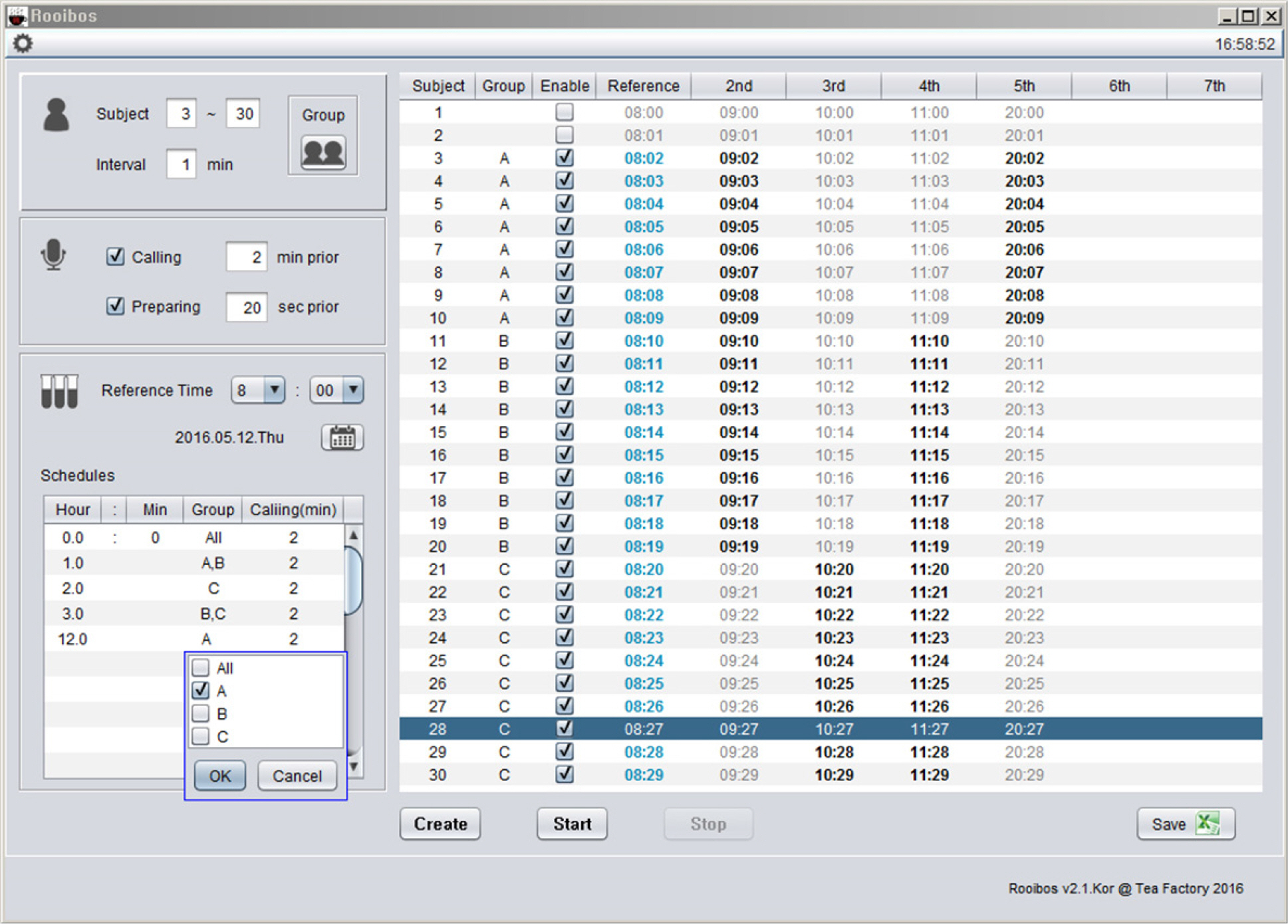
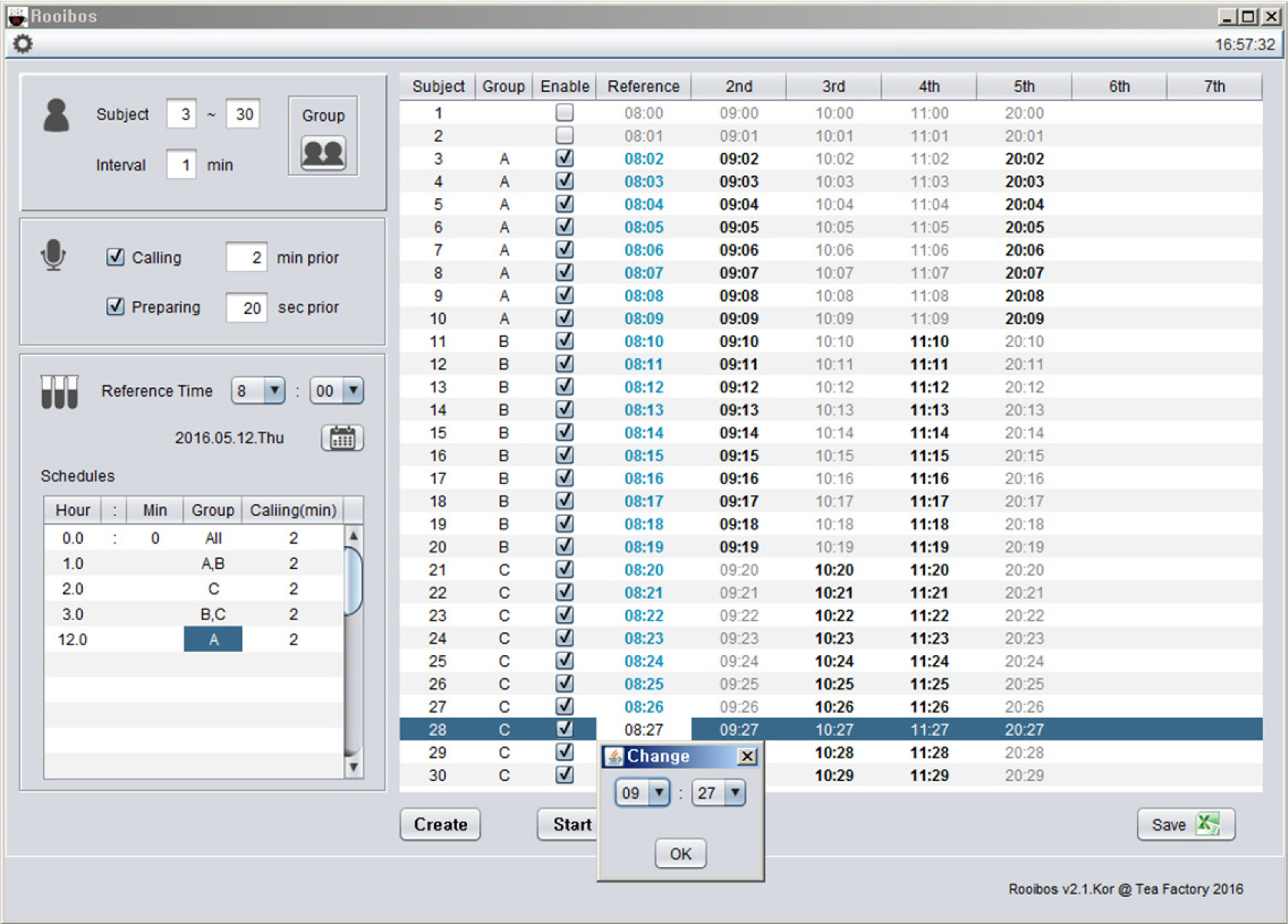
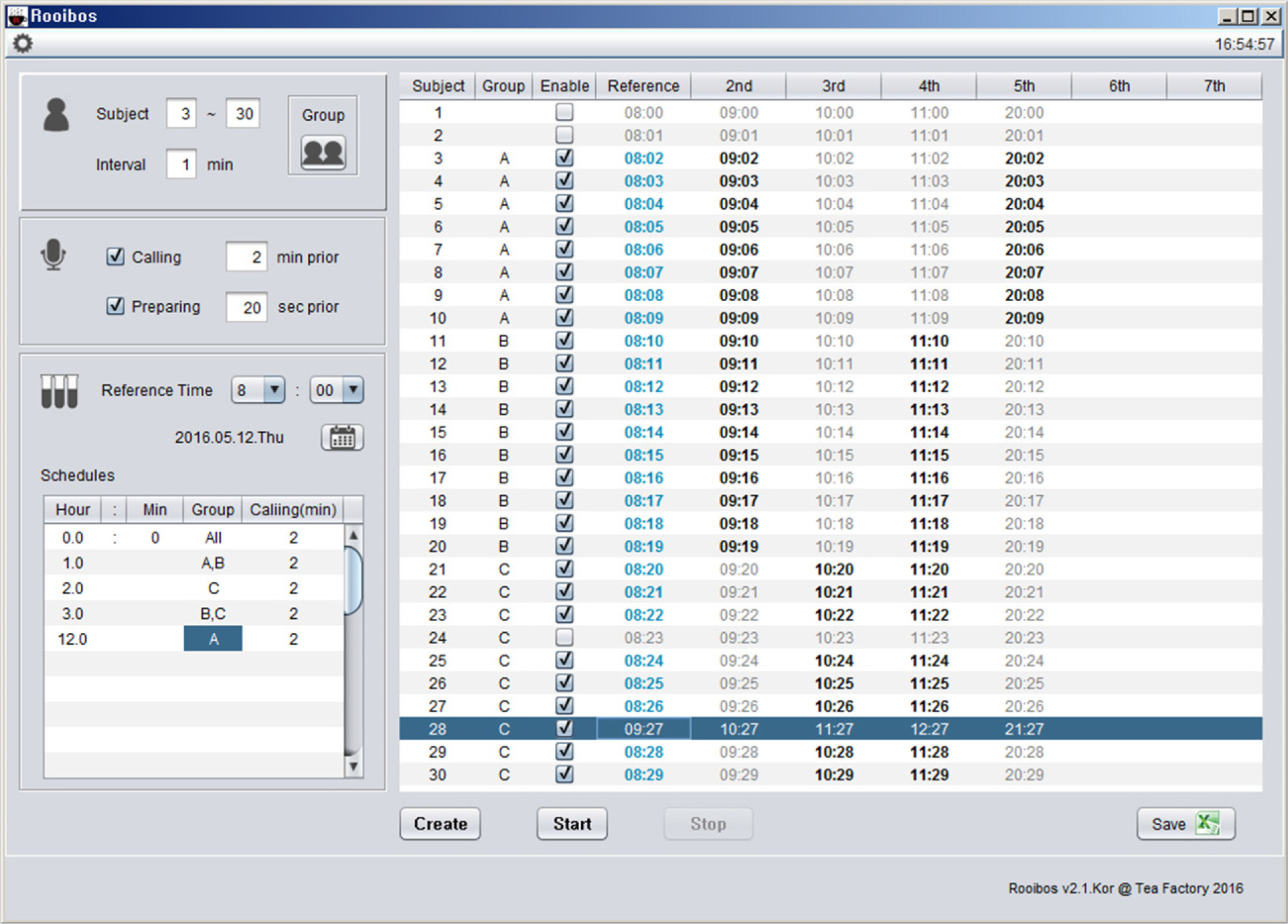
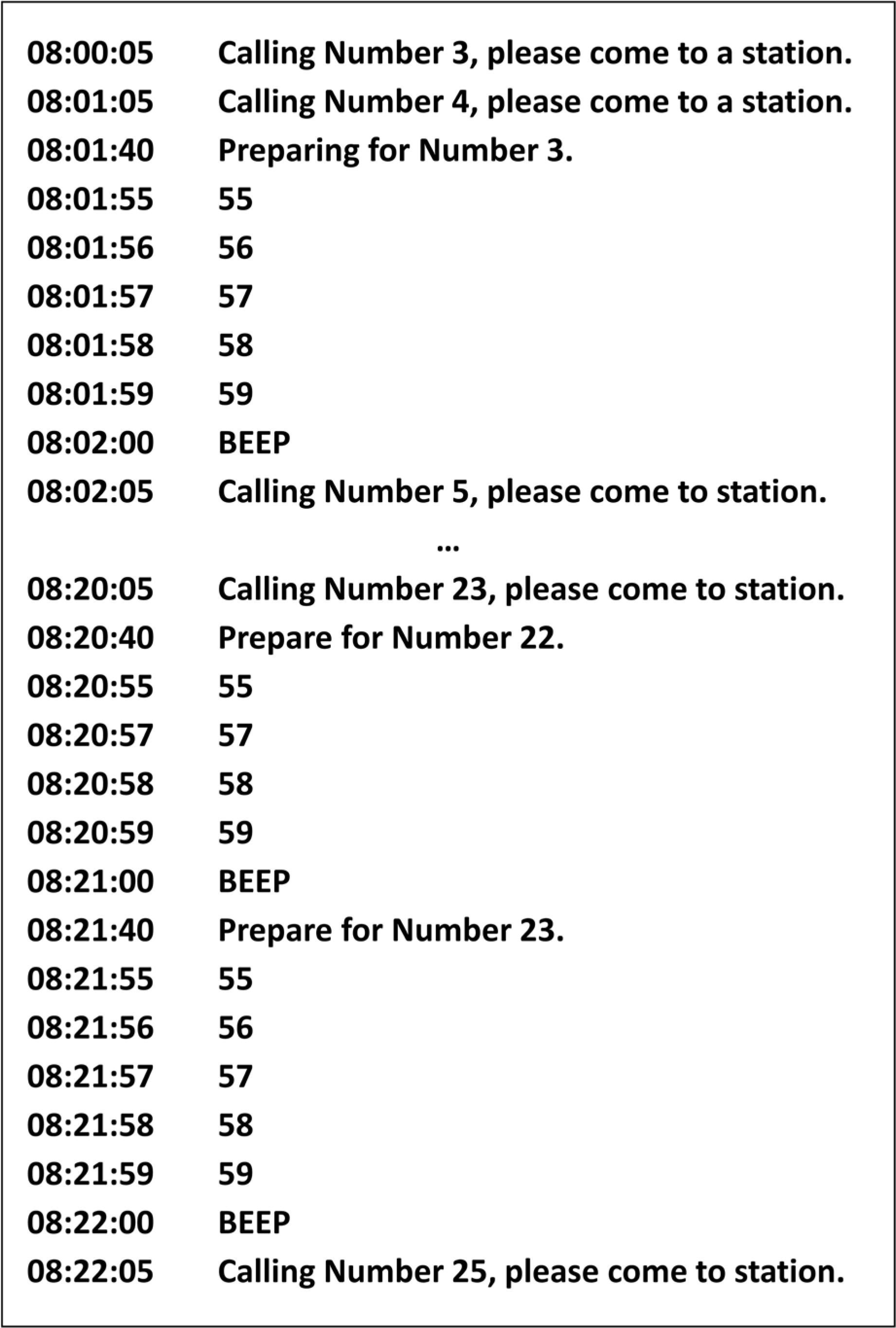
- Pharmacokinetic blood sampling is a prerequisite for successful early clinical trials. It is essential to take samples at the precise designated times to ensure the reliability of the clinical trial data; however, investigators have encountered difficulties in conducting procedures with limited manpower. We have recently developed automated schedule broadcast software (Rooibosâ„¢) to manage the precise scheduling of procedures for clinical trial centers. Rooibosâ„¢ is platform independent because it is programmed in the Java language. It generates scheduled times based on a reference time. It alarms at the scheduled times and pages subjects and alerts staff to prepare for the upcoming procedures. Rooibosâ„¢ can also group subjects when multiple clinical trials are conducted simultaneously in one or more clinical trial wards. This software may be applied to any study involving procedures that must be performed at designated times.
Figure
Reference
-
1.International Conference on Harmonisation. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice: E6. http://www.ich.org/fil-eadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed July. 2015.2.National Library of Medicine. Clinical Trials. https://medlineplus.gov/clini-caltrials.html. Accessed July. 2015.3.Meibohm B., Derendorf H. Pharmacokinetic/pharmacodynamic studies in drug product development. J Pharm Sci. 2002. 91:18–31.
Article4.US Food and Drug Administration. Guidance for Industry: Clinical Pharmacology Data to Support a Demonstration of Biosimilarity to a Reference Product. 2014. 436–261. http://www.fda.gov/downloads/drugs/guidancecomplian-ceregulatoryinformation/guidances/ucm397017.pdf. Accessed July 2015.5.Teuscher N. Using sampling “windows” for PK blood samples. http://learn-pkpd.com/2013/10/15/using-sampling-windows-for-pk-blood-samples/. Accessed Auguest. 2015.6.Zuckerman AE. Using the Java language to develop computer based patient records for use on the Internet. Proc AMIA Annu Fall Symp. 1996. 772–776.7.Choi HG., Jeon JY., Im YJ., Kim Y., Song EK., Seo YH, et al. Pharmacokinetic properties of two erlotinib 150 mg formulations with a genetic effect evaluation in healthy Korean subjects. Clin Drug Investig. 2015. 35:31–43. DOI: doi: 10.1007/s40261-014-0248-4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Development of Automated Bed-allocation Expert System in Clinical Research Ward
- Comparative accuracy of artificial intelligence-based AudaxCeph software, Dolphin software, and the manual technique for orthodontic landmark identification and tracing of lateral cephalograms
- Digital therapeutics and clinical pharmacology
- Time Efficiency and Diagnostic Accuracy of New Automated Myocardial Perfusion Analysis Software in 320-Row CT Cardiac Imaging
- Mammography with a fully automated breast volumetric software as a novel method for estimating the preoperative breast volume prior to mastectomy