Allergy Asthma Respir Dis.
2016 Jul;4(4):264-270. 10.4168/aard.2016.4.4.264.
Association of TLR3 gene polymorphism with IgG subclass deficiency and the severity in patients with aspirin-intolerant asthma
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Clinical Trial Center, Ajou University Hospital, Suwon, Korea.
- KMID: 2346755
- DOI: http://doi.org/10.4168/aard.2016.4.4.264
Abstract
- PURPOSE
Toll-like receptor 3 (TLR3) recognizes to viral double-stranded RNA and is involved in antiviral defenses. A probable role of TLR3 gene variants in the pathogenesis of aspirin-intolerant asthma (AIA) has been suggested. AIA patients present more frequent asthma exacerbations in which respiratory viral infections could be an exacerbating factor. IgG subclass deficiency was commonly present with bronchial asthma. Based on previous findings, we investigated whether TLR3 variants could affect IgG3 subclass deficiency in AIA.
METHODS
We enrolled 279 AIA patients, 403 aspirin-tolerant asthma (ATA) patients, and 315 normal healthy controls (NC) in this study. TLR3 polymorphism at the promoter region -299698G>T was genotyped. The serum levels of IgG subclasses were determined by the single radial immunodiffusion method. Expressions of IgG3 and TLR3 on Epstein-Barr virus transformed-B cells isolated from asthmatic patients were evaluated by flow cytometry to investigate B-cell functions.
RESULTS
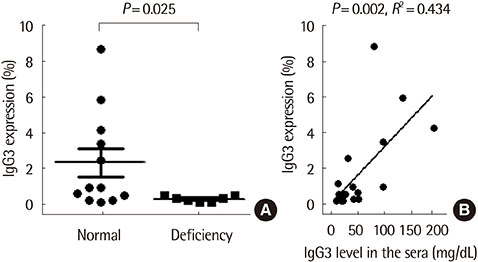
The TLR3 -299698 T allele was significantly associated with severity and IgG3 deficiency in the AIA group (P=0.044 and P=0.010, respectively), but not in the ATA group. IgG3 expression on B cells from asthmatics with IgG3 deficiency was significantly lower compared to those without (P=0.025). There was a positive correlation between IgG3 expression levels on B cells and serum IgG3 levels (r 2=0.434, P=0.002).
CONCLUSION
These results suggest that the TLR3 -299698G>T polymorphism may be associated with IgG3 subclass deficiency and severity in AIA.
MeSH Terms
Figure
Reference
-
1. Lee RU, Stevenson DD. Aspirin-exacerbated respiratory disease: evaluation and management. Allergy Asthma Immunol Res. 2011; 3:3–10.
Article2. Holgate ST. Mechanisms of asthma and implications for its prevention and treatment: a personal journey. Allergy Asthma Immunol Res. 2013; 5:343–347.
Article3. Szczeklik A. Aspirin-induced asthma as a viral disease. Clin Allergy. 1988; 18:15–20.
Article4. Szczeklik A, Stevenson DD. Aspirin-induced asthma: advances in pathogenesis, diagnosis, and management. J Allergy Clin Immunol. 2003; 111:913–921.
Article5. Szczeklik A, Schmitz-Schumann M, Nizankowska E, Milewski M, Roehlig F, Virchow C. Altered distribution of IgG subclasses in aspirin-induced asthma: high IgG4, low IgG1. Clin Exp Allergy. 1992; 22:283–287.
Article6. Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006; 117:979–987.
Article7. Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004; 5:975–979.
Article8. Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, et al. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol. 2008; 181:276–287.
Article9. Sackesen C, van de Veen W, Akdis M, Soyer O, Zumkehr J, Ruckert B, et al. Suppression of B-cell activation and IgE, IgA, IgG1 and IgG4 production by mammalian telomeric oligonucleotides. Allergy. 2013; 68:593–603.
Article10. Park SM, Park JS, Park HS, Park CS. Unraveling the genetic basis of aspirin hypersensitivity in asthma beyond arachidonate pathways. Allergy Asthma Immunol Res. 2013; 5:258–276.
Article11. Palikhe NS, Kim SH, Kim JH, Losol P, Ye YM, Park HS. Role of toll-like receptor 3 variants in aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2011; 3:123–127.
Article12. Daley D, Park JE, He JQ, Yan J, Akhabir L, Stefanowicz D, et al. Associations and interactions of genetic polymorphisms in innate immunity genes with early viral infections and susceptibility to asthma and asthma-related phenotypes. J Allergy Clin Immunol. 2012; 130:1284–1293.
Article13. Sironi M, Biasin M, Cagliani R, Forni D, De Luca M, Saulle I, et al. A common polymorphism in TLR3 confers natural resistance to HIV-1 infection. J Immunol. 2012; 188:818–823.
Article14. Lim MS, Elenitoba-Johnson KS. The molecular pathology of primary immunodeficiencies. J Mol Diagn. 2004; 6:59–83.
Article15. Popa V, Nagy SM Jr. Immediate hypersensitivity in adults with IgG deficiency and recurrent respiratory infections. Ann Allergy Asthma Immunol. 1999; 82:567–573.
Article16. Kim JH, Park HJ, Choi GS, Kim JE, Ye YM, Nahm DH, et al. Immunoglobulin G subclass deficiency is the major phenotype of primary immunodeficiency in a Korean adult cohort. J Korean Med Sci. 2010; 25:824–828.
Article17. Abrahamian F, Agrawal S, Gupta S. Immunological and clinical profile of adult patients with selective immunoglobulin subclass deficiency: response to intravenous immunoglobulin therapy. Clin Exp Immunol. 2010; 159:344–350.
Article18. de Moraes, Oliveira LC, Diogo CL, Kirschfink M, Grumach AS. Immunoglobulin G subclass concentrations and infections in children and adolescents with severe asthma. Pediatr Allergy Immunol. 2002; 13:195–202.
Article19. Loftus BG, Price JF, Lobo-Yeo A, Vergani D. IgG subclass deficiency in asthma. Arch Dis Child. 1988; 63:1434–1437.
Article20. Soderström T, Soderström R, Avanzini A, Brandtzaeg P, Karlsson G, Hanson LA. Immunoglobulin G subclass deficiencies. Int Arch Allergy Appl Immunol. 1987; 82:476–480.
Article21. Kim YJ, Lim KH, Kim MY, Jo EJ, Lee SY, Lee SE, et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol Res. 2014; 6:156–162.
Article22. Proceedings of. current understanding, recommendations, and unanswered questions. American Thoracic Society. Am J Respir Crit Care Med. 2000; 162:2341–2351.23. Hopkins C, Browne JP, Slack R, Lund V, Brown P. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict? Otolaryngol Head Neck Surg. 2007; 137:555–561.
Article24. Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy Asthma Immunol. 2007; 99:281–283.
Article25. Jolliff CR, Cost KM, Stivrins PC, Grossman PP, Nolte CR, Franco SM, et al. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin Chem. 1982; 28:126–128.
Article26. Tosato G, Cohen JI. Generation of Epstein-barr virus (EBV)-immortalized B cell lines. Curr Protoc Immunol. 2007; Chapter 7:Unit 7.22.
Article27. Skvaril F. IgG subclasses in viral infections. Monogr Allergy. 1986; 19:134–143.28. Hanson LA, Soderstrom R, Avanzini A, Bengtsson U, Bjorkander J, Soderstrom T. Immunoglobulin subclass deficiency. Pediatr Infect Dis J. 1988; 7:5 Suppl. S17–S21.29. Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013; 33:195–210.
Article30. Edelmann KH, Richardson-Burns S, Alexopoulou L, Tyler KL, Flavell RA, Oldstone MB. Does Toll-like receptor 3 play a biological role in virus infections? Virology. 2004; 322:231–238.
Article31. Ranjith-Kumar CT, Miller W, Sun J, Xiong J, Santos J, Yarbrough I, et al. Effects of single nucleotide polymorphisms on Toll-like receptor 3 activity and expression in cultured cells. J Biol Chem. 2007; 282:17696–17705.
Article32. Yang HY, Lee HS, Lee CH, Fang WH, Chen HC, Salter DM, et al. Association of a functional polymorphism in the promoter region of TLR-3 with osteoarthritis: a two-stage case-control study. J Orthop Res. 2013; 31:680–685.
Article33. Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004; 172:6065–6073.
Article34. Simone R, Floriani A, Saverino D. Stimulation of human CD4+ T lymphocytes via TLR3, TLR5 and TLR7/8 up-regulates expression of costimulatory and modulates proliferation. Open Microbiol J. 2009; 3:1–8.
Article35. Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005; 438:364–368.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of intravenous immunoglobulin therapy in severe aspirin - sensitive asthma patient combined with IgG1 and IgG3 subclass deficiency
- Serum IgG and IgG subclass in aspirin-sensitive asthma
- Serum IgG and IgG subclass in bronchial asthma
- Diagnosis and treatment of immunoglobulin G subclass deficiency in a school-age child with recurrent wheezing
- Role of Toll-like Receptor 3 Variants in Aspirin-Exacerbated Respiratory Disease