J Gynecol Oncol.
2015 Oct;26(4):327-335. 10.3802/jgo.2015.26.4.327.
Identification of high-affinity VEGFR3-binding peptides through a phage-displayed random peptide library
- Affiliations
-
- 1Department of Obstetrics and Gynecology, No.117 Center Military Hospital, Hangzhou, China. lifeng_shi@163.com
- KMID: 2345924
- DOI: http://doi.org/10.3802/jgo.2015.26.4.327
Abstract
OBJECTIVE
Vascular endothelial growth factor (VEGF) interaction with its receptor, VEGFR-3/Flt-4, regulates lymphangiogenesis. VEGFR-3/Flt-4 expression in cancer cells has been correlated with clinical stage, lymph node metastasis, and lymphatic invasion. The objective of this study is to identify a VEGFR-3/Flt-4-interacting peptide that could be used to inhibit VEGFR-3 for ovarian cancer therapy.
METHODS
The extracellular fragment of recombinant human VEGFR-3/Flt-4 (rhVEGFR-3/Flt-4) fused with coat protein pIII was screened against a phage-displayed random peptide library. Using affinity enrichment and enzyme-linked immunosorbent assay (ELISA) screening, positive clones of phages were amplified. Three phage clones were selected after four rounds of biopanning, and the specific binding of the peptides to rhVEGFR-3 was detected by ELISA and compared with that of VEGF-D. Immunohistochemistry and immunofluorescence analyses of ovarian cancer tissue sections was undertaken to demonstrate the specificity of the peptides.
RESULTS
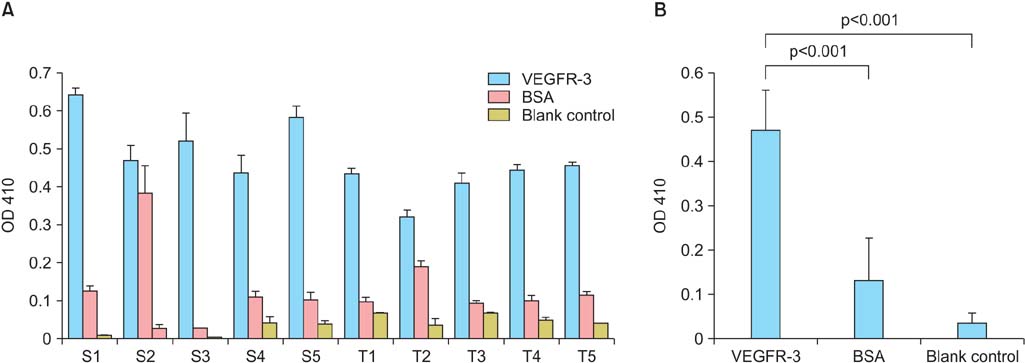
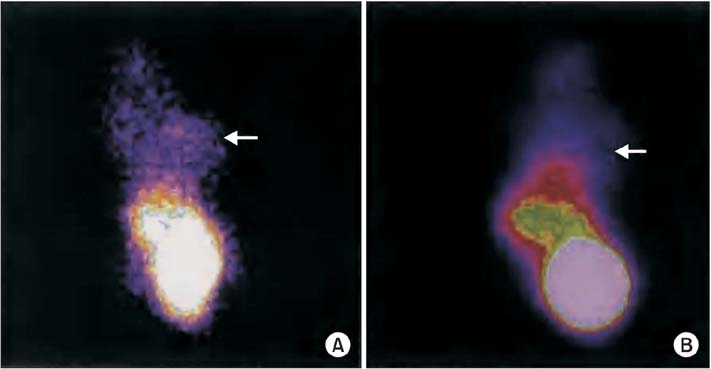
After four rounds of biopanning, ELISA confirmed the specificity of the enriched bound phage clones for rhVEGFR-3. Sequencing and translation identified three different peptides. Non-competitive ELISA revealed that peptides I, II, and III had binding affinities for VEGFR-3 with Kaff (affinity constant) of 16.4+/-8.6 microg/mL (n=3), 9.2+/-2.1 microg/mL (n=3), and 174.8+/-31.1 microg/mL (n=3), respectively. In ovarian carcinoma tissue sections, peptide III (WHWLPNLRHYAS), which had the greatest binding affinity, also co-localized with VEGFR-3 in endothelial cells lining lymphatic vessels; its labeling of ovarian tumors in vivo was also confirmed.
CONCLUSION
These finding showed that peptide III has high specificity and activity and, therefore, may represent a potential therapeutic approach to target VEGF-VEGFR-3 signaling for the treatment or diagnosis of ovarian cancer.
Keyword
MeSH Terms
-
Enzyme-Linked Immunosorbent Assay
Female
Humans
Ovarian Neoplasms/*therapy
*Peptide Library
Sequence Analysis, DNA
Signal Transduction/physiology
Vascular Endothelial Growth Factor A/metabolism
Vascular Endothelial Growth Factor Receptor-3/antagonists & inhibitors/*metabolism
Peptide Library
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor Receptor-3
Figure
Reference
-
1. Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000; 50:7–33.2. Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003; 3:502–516.3. Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009; 45:931–991.4. Della Pepa C, Banerjee S. Bevacizumab in combination with chemotherapy in platinum-sensitive ovarian cancer. Onco Targets Ther. 2014; 7:1025–1032.5. Al-Rawi MA, Mansel RE, Jiang WG. Molecular and cellular mechanisms of lymphangiogenesis. Eur J Surg Oncol. 2005; 31:117–121.6. Ding MX, Li JC, Tang XH. Lymphatic metastasis and VEGF-C expression in ovarian epithelial tumors. Shi Yan Sheng Wu Xue Bao. 2003; 36:445–452.7. Zhang S, Yu H, Zhang L. Role of vascular endothelial growth factor receptor-3/Flt-4 in early-stage cervical cancer. Oncol Lett. 2010; 1:453–456.8. Omoto I, Matsumoto M, Okumura H, Uchikado Y, Setoyama T, Kita Y, et al. Expression of vascular endothelial growth factor-C and vascular endothelial growth factor receptor-3 in esophageal squamous cell carcinoma. Oncol Lett. 2014; 7:1027–1032.9. Qiu H, Cao L, Wang D, Xu H, Liang Z. High levels of circulating CD34+/VEGFR3+ lymphatic/vascular endothelial progenitor cells is correlated with lymph node metastasis in patients with epithelial ovarian cancer. J Obstet Gynaecol Res. 2013; 39:1268–1275.10. Lim JJ, Yang K, Taylor-Harding B, Wiedemeyer WR, Buckanovich RJ. VEGFR3 inhibition chemosensitizes ovarian cancer stemlike cells through down-regulation of BRCA1 and BRCA2. Neoplasia. 2014; 16:343–353.e2.11. Underiner TL, Ruggeri B, Gingrich DE. Development of vascular endothelial growth factor receptor (VEGFR) kinase inhibitors as anti-angiogenic agents in cancer therapy. Curr Med Chem. 2004; 11:731–745.12. He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002; 94:819–825.13. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011; 365:2473–2483.14. Gordon MS, Cunningham D. Managing patients treated with bevacizumab combination therapy. Oncology. 2005; 69:Suppl 3. 25–33.15. Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis. JAMA. 2008; 300:2277–2285.16. Huang H, Zheng Y, Zhu J, Zhang J, Chen H, Chen X. An updated meta-analysis of fatal adverse events caused by bevacizumab therapy in cancer patients. PLoS One. 2014; 9:e89960.17. Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, et al. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006; 66:2650–2657.18. Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, et al. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005; 65:6901–6909.19. Rinderknecht M, Villa A, Ballmer-Hofer K, Neri D, Detmar M. Phage-derived fully human monoclonal antibody fragments to human vascular endothelial growth factor-C block its interaction with VEGF receptor-2 and 3. PLoS One. 2010; 5:e11941.20. Lamdan H, Gavilondo JV, Munoz Y, Pupo A, Huerta V, Musacchio A, et al. Affinity maturation and fine functional mapping of an antibody fragment against a novel neutralizing epitope on human vascular endothelial growth factor. Mol Biosyst. 2013; 9:2097–2106.21. Erdag B, Balcioglu KB, Kumbasar A, Celikbicak O, Zeder-Lutz G, Altschuh D, et al. Novel short peptides isolated from phage display library inhibit vascular endothelial growth factor activity. Mol Biotechnol. 2007; 35:51–63.22. Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, et al. Single-chain antigen-binding proteins. Science. 1988; 242:423–426.23. Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992; 52:3402–3408.24. Mayer A, Chester KA, Flynn AA, Begent RH. Taking engineered anti-CEA antibodies to the clinic. J Immunol Methods. 1999; 231:261–273.25. Akabani G, McLendon RE, Bigner DD, Zalutsky MR. Vascular targeted endoradiotherapy of tumors using alpha-particle-emitting compounds: theoretical analysis. Int J Radiat Oncol Biol Phys. 2002; 54:1259–1275.26. Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press;2001.27. Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987; 100:173–179.28. Winnard P Jr, Chang F, Rusckowski M, Mardirossian G, Hnatowich DJ. Preparation and use of NHS-MAG3 for technetium-99m labeling of DNA. Nucl Med Biol. 1997; 24:425–432.29. Kato S, Shimoda H, Ji RC, Miura M. Lymphangiogenesis and expression of specific molecules as lymphatic endothelial cell markers. Anat Sci Int. 2006; 81:71–83.30. Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, Carbone M, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999; 81:54–61.31. Trepel M, Arap W, Pasqualini R. In vivo phage display and vascular heterogeneity: implications for targeted medicine. Curr Opin Chem Biol. 2002; 6:399–404.32. Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006; 58:1622–1654.33. Qin X, Wan Y, Li M, Xue X, Wu S, Zhang C, et al. Identification of a novel peptide ligand of human vascular endothelia growth factor receptor 3 for targeted tumour diagnosis and therapy. J Biochem. 2007; 142:79–85.34. Smith GP, Petrenko VA. Phage display. Chem Rev. 1997; 97:391–410.35. Capiaumont J, Jacob C, Sarem M, Nabet P, Belleville F, Dousset B. Assay of a seric human hexapeptide (HWESAS) using a monoclonal antibody and ELISA. Clin Chim Acta. 2000; 293:89–103.36. Barkia A, Martin C, Puchois P, Gesquiere JC, Cachera C, Tartar A, et al. Enzyme-linked immunosorbent assay for human proapolipoprotein A-I using specific antibodies against synthetic peptide. J Lipid Res. 1988; 29:77–84.37. Pesce AJ, Michael JG. Artifacts and limitations of enzyme immunoassay. J Immunol Methods. 1992; 150:111–119.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Definition of the peptide mimotope of cellular receptor for hepatitis C virus E2 protein using random peptide library
- A Phage Display-Identified Peptide Selectively Binds to Kidney Injury Molecule-1 (KIM-1) and Detects KIM-1–Overexpressing Tumors In Vivo
- Screening of Peptide Libraries to Investigate the Substrate Specificity of UL97 Protein Kinase from Human Cytomegalovirus
- Priming of Autoreactive CD8+ T Cells Is Inhibited by Immunogenic Peptides Which Are Competitive for Major Histocompatibility Complex Class I Binding
- Engineering a High-Affinity PD-1 Peptide for Optimized Immune Cell-Mediated Tumor Therapy