J Bacteriol Virol.
2006 Jun;36(2):119-124. 10.4167/jbv.2006.36.2.119.
Screening of Peptide Libraries to Investigate the Substrate Specificity of UL97 Protein Kinase from Human Cytomegalovirus
- Affiliations
-
- 1Department of Molecular Medicine, School of Medicine, Kyungpook National University, Daegu, Korea. mcbaek@knu.ac.kr
- KMID: 2055018
- DOI: http://doi.org/10.4167/jbv.2006.36.2.119
Abstract
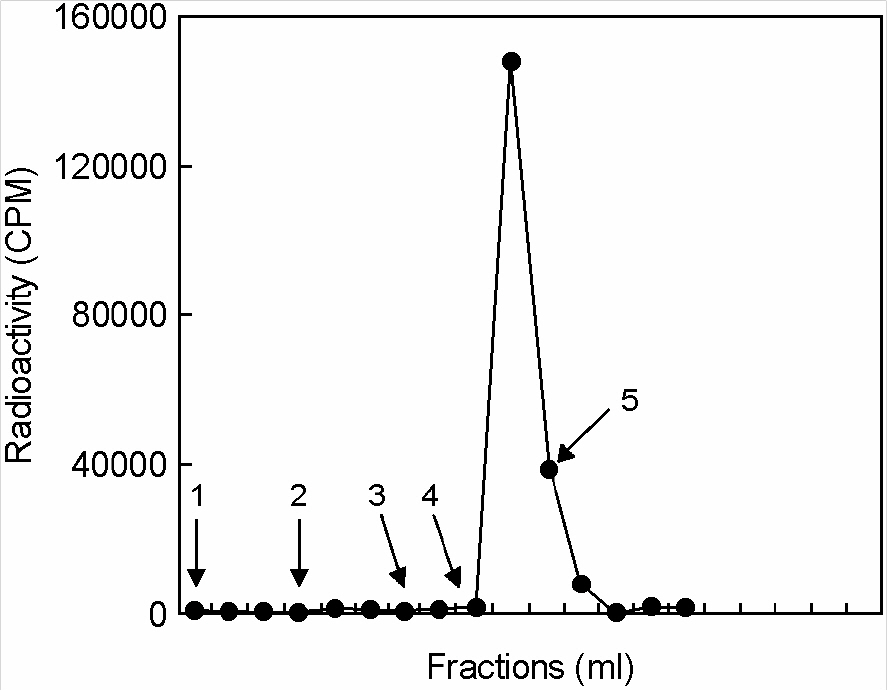
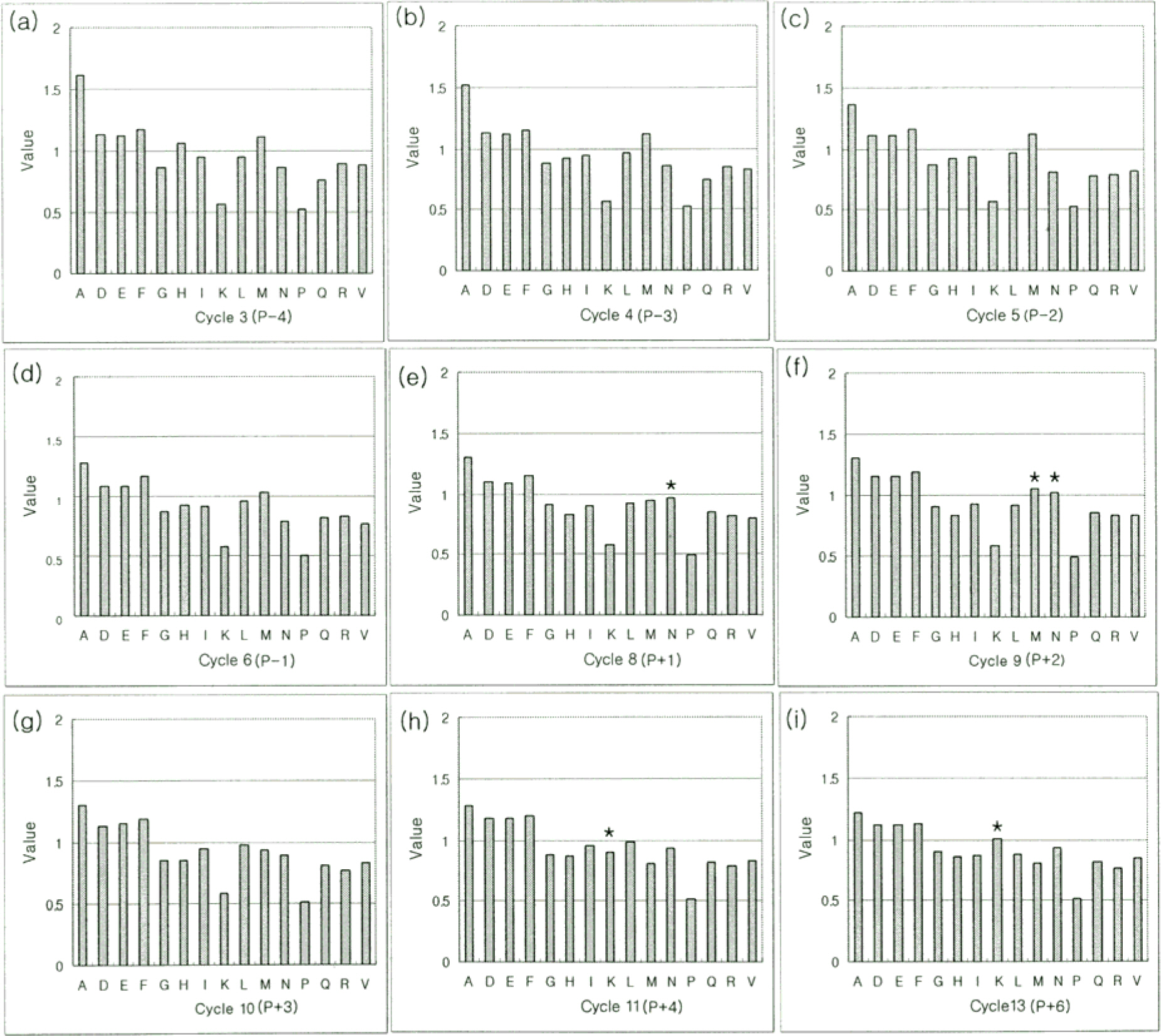
- Human cytomegalovirus encodes an unusual protein kinase UL97 which can phosphorylate exogenous substrates, including histone H2B and nucleoside analogs such as ganciclovir. The previous result interestingly showed that the peptides phosphorylated by UL97 have K/R at the 5 positions (P+5) downstream from the pSer. To confirm the importance of the basic residue in the position, we used two peptide libraries, 4S4K (MAXXXXSXXXXKXANNN) and 4S6N (MAXXXXSXXXXXXNNN). The activity of phosphorylation by UL97 was higher in the peptide library 4S4K than 4S6N, suggesting the importance of basic residue at P+5 position. The screening with a peptide library 4S4K showed slight tendencies for N in the P+1 and P+2, M in the P+2, K in the P+4 and P+6 positions and several amino acids in the other positions. This result will give information to develop an optimal peptide for screening a novel UL97 inhibitor.
MeSH Terms
Figure
Cited by 1 articles
-
Human Cytomegalovirus Infection in Solid-Organ Transplantation
Yong-Hee Kim
J Bacteriol Virol. 2015;45(1):11-18. doi: 10.4167/jbv.2015.45.1.11.
Reference
-
References
1). Baek MC, Krosky PM, Coen DM. Relationship between autophosphorylation and phosphorylation of exogenous substrates by the human cytomegalovirus UL97 protein kinase. J Virol. 76:11943–11952. 2002.
Article2). Baek MC, Krosky PM, He Z, Coen DM. Specific phosphorylation of exogenous protein and peptide substrates by the human cytomegalovirus UL97 protein kinase. Importance of the P+5 position. J. Biol. Chem. 277:29593–29599. 2002.3). Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA 3rd, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. Potent and selective inhibition of human cytome-galovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 46:2365–2372. 2002.4). Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA 3rd, Davis MG, Talarico CL, Miller WH, Ferris R, Dornsife RE, Stanat SC, Drach JC, Townsend LB, Koszalka GW. Interactions of 1263W94 with other antiviral agents in inhibition of human cytomegalovirus replication. Antimicrob Agents Chemother. 47:1468–1471. 2003.
Article5). Chee MS, Lawrence GL, Barrell BG. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J Gen Virol. 70:1151–1160. 1989.
Article6). Crossen R., Gruenwald S. In. Baculovirus Expression Vector System Manual. 5th ed.Pharmingen;San Diego: 1998.7). Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576–596. 1995.8). Hayden FG. Antiviral agents (nonretroviral). pp.p. 1313–1347. In. Goodman & Gilman's The pharmacological basis of therapeutics. 10th ed.Hardman J.G., Limbird L.E., Gilman A. G., editors(Ed.),. McGraw-Hill;New York, N.Y.: 2001.9). He Z, He YS, Kim Y, Chu L, Ohmstede C, Biron KK, Coen DM. The human cytomegalovirus UL97 protein is a protein kinase that autophosphorylates on serines and threonines. J Virol. 71:405–411. 1997.
Article10). Johnson LN, Lowe ED, Noble ME, Owen DJ. The Eleventh Datta Lecture. The structural basis for substrate recognition and control by protein kinases. FEBS Lett. 430:1–11. 1998.11). Johnson LN, Noble ME, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 85:149–158. 1996.
Article12). Krosky PM, Baek MC, Coen DM. The human cytomegalo-virus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 77:905–914. 2003.
Article13). Krosky PM, Baek MC, Jahng WJ, Barrera I, Harvey RJ, Biron KK, Coen DM, Sethna PB. The human cytomegalo-virus UL44 protein is a substrate for the UL97 protein kinase. J Virol. 77:7720–7727. 2003.
Article14). Littler E, Stuart AD, Chee MS. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 358:160–162. 1992.
Article15). McGeoch DJ, Coulter LJ, Moss HWM. Vol. 1:pp.p. 391–393. In. The Protein Kinase Facts Book. Hardie G, Hanks S, editors. (Ed),. Academic Press;London: 1995.16). Prichard MN, Gao N, Jairath S, Mulamba G, Krosky P, Coen DM, Parker BO, Pari GS. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J Virol. 73:5663–5670. 1999.17). Selleseth DW, Talarico CL, Miller T, Lutz MW, Biron KK, Harvey RJ. In vitro activities of benzimidazole D- and L-ribonucleosides against herpesviruses. Antimicrob Agents Chemother. 47:2186–2192. 2003.18). Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol. 4:973–982. 1994.
Article19). Songyang Z, Cantley LC. The use of peptide library for the determination of kinase peptide substrates. Methods Mol Biol. 87:87–98. 1998.
Article20). Songyang Z, Lu KP, Kwon YT, Tsai L-H, Filhol O, Cochet C, Brickey DA, Soderling TR, Bartleson C, Graves DJ, Demaggio AJ, Hoekstra MF, Blenis J, Hunter T, Cantley LC. A structural basis for substrate specificities of protein Ser/Thr kinases: primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk. Mol. Cell. Biol. 16:6486–6493. 1996.21). Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 358:162–164. 1992.
Article22). Talarico CL, Burnette TC, Miller WH, Smith SL, Davis MG, Stanat SC, Ng TI, He Z, Coen DM, Roizman B, Biron KK. Acyclovir is phosphorylated by the human cytomegalovirus UL97 protein. Antimicrob Agents Chemother. 43:1941–1946. 1999.
Article23). Taylor SS, Radzio-Andzelm E, Hunter T. How do protein kinases discriminate between serine/threonine and tyrosine? Structural insights from the insulin receptor protein-tyrosine kinase. FASEB J. 9:1255–1266. 1995.
Article24). Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc Natl Acad Sci U S A. 98:1895–1900. 2001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiviral resistance in human cytomegalovirus due to UL54 mutations without UL97 mutations in Korea
- The First Case of Ganciclovir-Resistant Cytomegalovirus Colitis with a 597-600 Deletion in UL97 Gene after Stem Cell Transplantation in Korea
- Identification of novel substrates for human checkpoint kinase Chk1 and Chk2 through genome-wide screening using a consensus Chk phosphorylation motif
- A Case of Ganciclovir-resistant CMV Antigenemia by UL97 Phosphotransferase Mutant Strain after Cord Blood Transplantation
- Evaluation of a tyrosine kinase peptide microarray for tyrosine kinase inhibitor therapy selection in cancer