Cancer Res Treat.
2022 Apr;54(2):362-374. 10.4143/crt.2021.424.
Engineering a High-Affinity PD-1 Peptide for Optimized Immune Cell-Mediated Tumor Therapy
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, Hospital of Stomatology, Guanghua School of Stomatology, Guangdong Provincial Key Laboratory of Stomatology, Sun Yat-sen University, Guangzhou, China
- 2Laboratory of Cancer and Stem Cell Biology, Key Laboratory of Gene Engineering of the Ministry of Education, State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou Higher Education Mega Center, Guangzhou, China
- 3Guangzhou Yidai Pharmaceutical Co., Ltd., Guangzhou, Guangdong, China
- KMID: 2528206
- DOI: http://doi.org/10.4143/crt.2021.424
Abstract
- Purpose
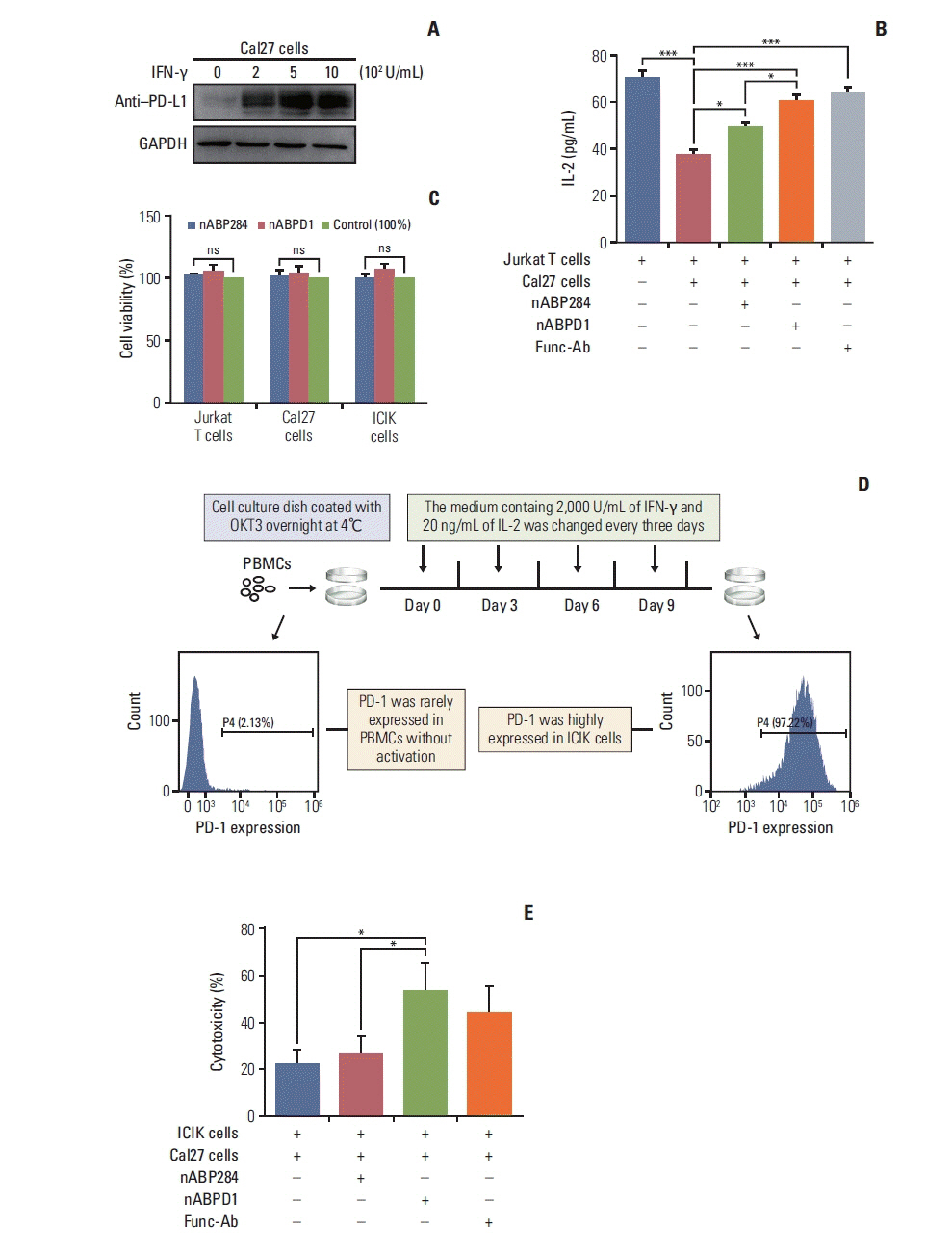
The purpose of this study was to optimize a peptide (nABP284) that binds to programmed cell death protein 1 (PD-1) by a computer-based protocol in order to increase its affinity. Then, this study aimed to determine the inhibitory effects of this peptide on cancer immune escape by coculturing improving cytokine-induced killer (ICIK) cells with cancer cells.
Materials and Methods
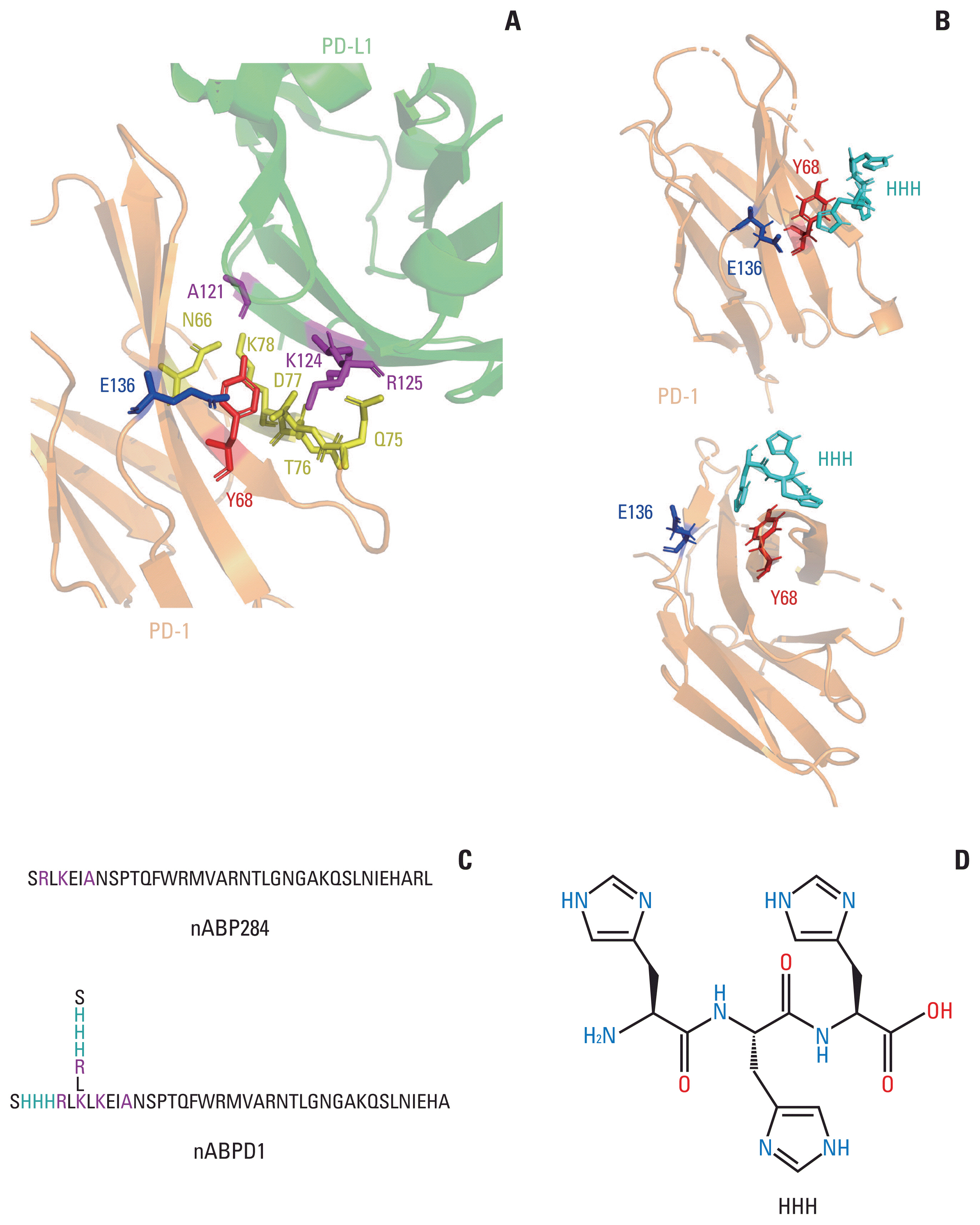
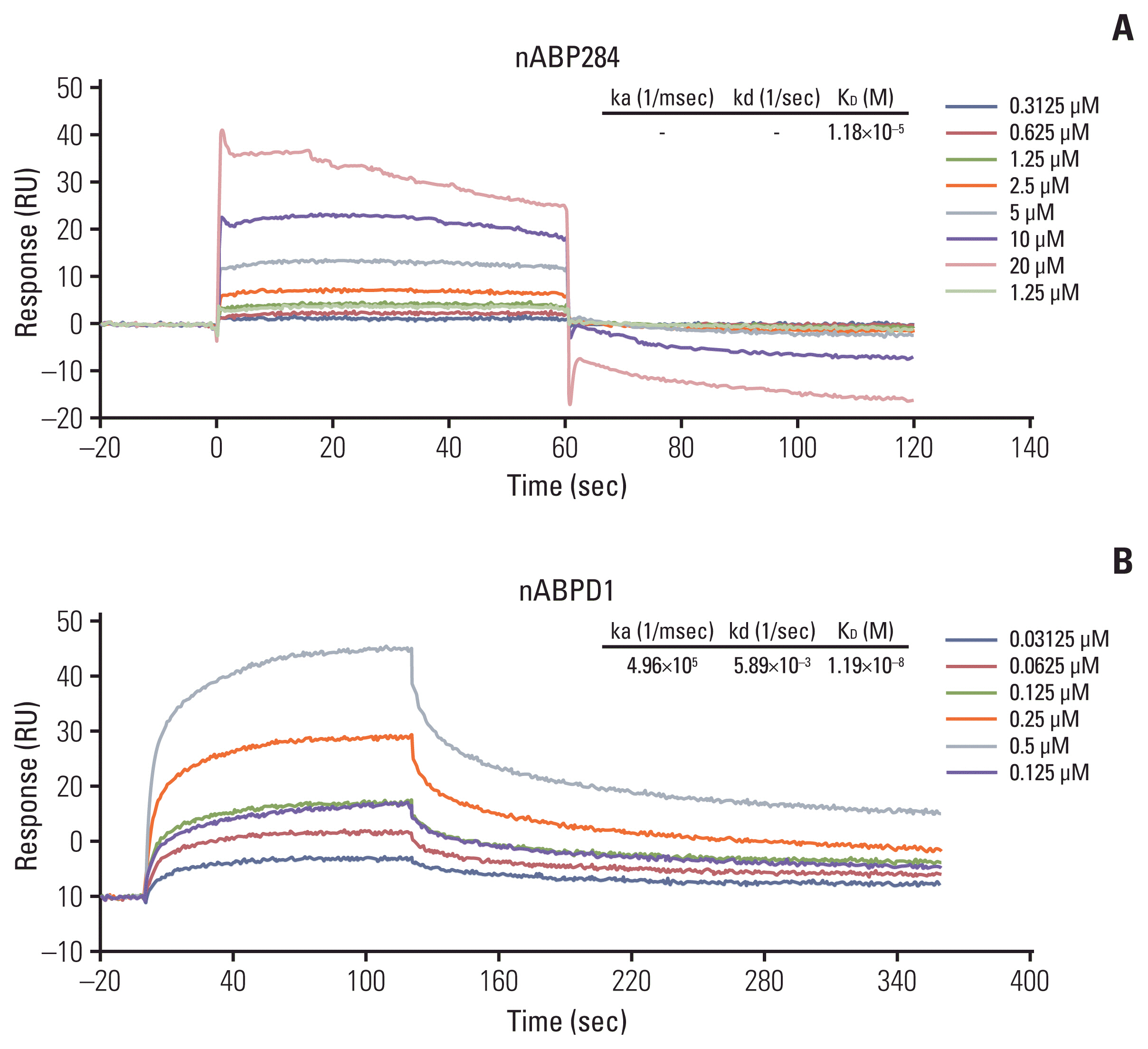
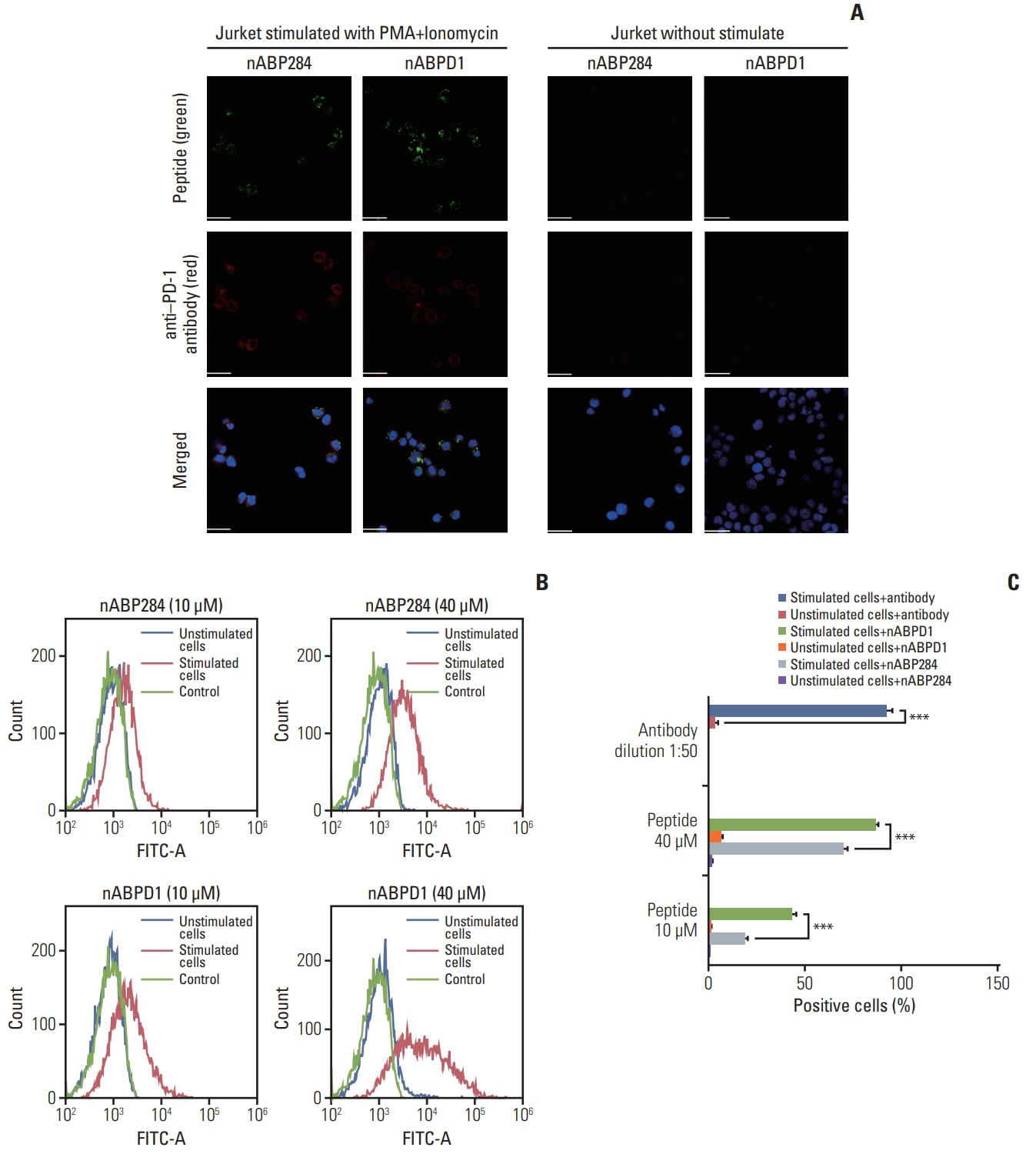
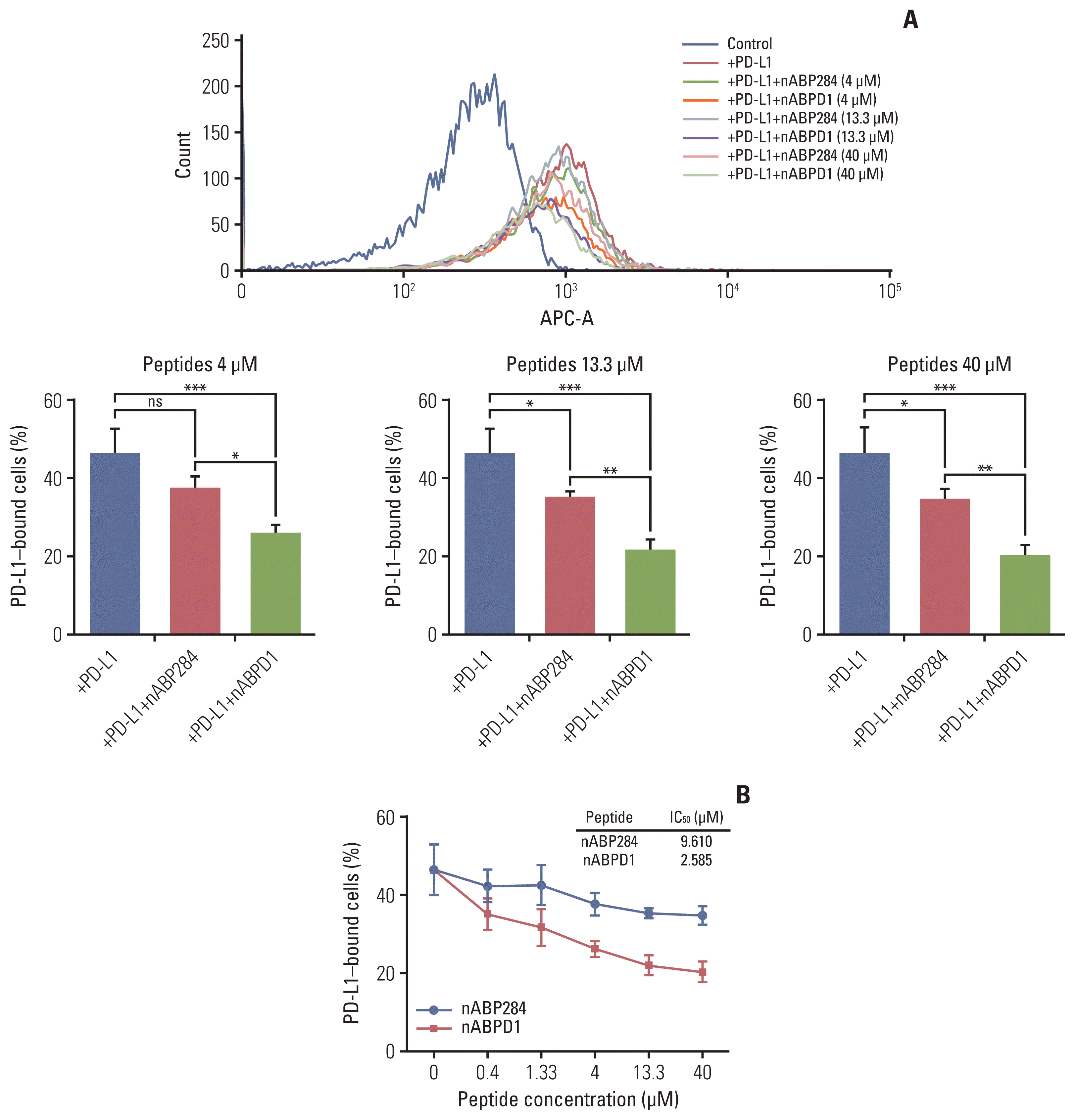
nABP284 that binds to PD-1 was identified by phage display technology in our previous study. AutoDock and PyMOL were used to optimize the sequence of nABP284 to design a new peptide (nABPD1). Immunofluorescence was used to demonstrate that the peptides bound to PD-1. Surface plasmon resonance was used to measure the binding affinity of the peptides. The blocking effect of the peptides on PD-1 was evaluated by a neutralization experiment with human recombinant programmed death-ligand 1 (PD-L1) protein. The inhibition of activated lymphocytes by cancer cells was simulated by coculturing of human acute T lymphocytic leukemia cells (Jurkat T cells) with human tongue squamous cell carcinoma cells (Cal27 cells). The anticancer activities were determined by coculturing ICIK cells with Cal27 cells in vitro.
Results
A high-affinity peptide (nABPD1, KD=11.9 nM) for PD-1 was obtained by optimizing the nABP284 peptide (KD=11.8 μM). nABPD1 showed better efficacy than nABP284 in terms of increasing the secretion of interkeulin-2 by Jurkat T cells and enhancing the in vitro antitumor activity of ICIK cells.
Conclusion
nABPD1 possesses higher affinity for PD-1 than nABP284, which significantly enhances its ability to block the PD-1/PD-L1 interaction and to increase ICIK cell-mediated antitumor activity by armoring ICIK cells.
Keyword
Figure
Reference
-
References
1. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019; 18:175–96.
Article2. Yang Y. Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest. 2015; 125:3335–7.
Article3. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008; 26:677–704.
Article4. Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015; 36:265–76.
Article5. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015; 27:450–61.
Article6. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015; 373:23–34.
Article7. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015; 372:2521–32.
Article8. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015; 372:2018–28.
Article9. Morgensztern D, Herbst RS. Nivolumab and pembrolizumab for non-small cell lung cancer. Clin Cancer Res. 2016; 22:3713–7.
Article10. Yang J, Hu L. Immunomodulators targeting the PD-1/PD-L1 protein-protein interaction: from antibodies to small molecules. Med Res Rev. 2019; 39:265–301.
Article11. Ruoslahti E. Peptides as targeting elements and tissue penetration devices for nanoparticles. Adv Mater. 2012; 24:3747–56.
Article12. Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015; 20:122–8.
Article13. Zak KM, Kitel R, Przetocka S, Golik P, Guzik K, Musielak B, et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure. 2015; 23:2341–8.
Article14. Lim H, Chun J, Jin X, Kim J, Yoon J, No KT. Investigation of protein-protein interactions and hot spot region between PD-1 and PD-L1 by fragment molecular orbital method. Sci Rep. 2019; 9:16727.
Article15. Biesiada J, Porollo A, Velayutham P, Kouril M, Meller J. Survey of public domain software for docking simulations and virtual screening. Hum Genomics. 2011; 5:497–505.
Article16. Martinez X, Krone M, Alharbi N, Rose AS, Laramee RS, O’Donoghue S, et al. Molecular graphics: bridging structural biologists and computer scientists. Structure. 2019; 27:1617–23.
Article17. Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, et al. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res. 2014; 20:3003–11.
Article18. Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015; 148:1383–91.
Article19. Huang X, Zhang J, Li X, Huang H, Liu Y, Yu M, et al. Rescue of iCIKs transfer from PD-1/PD-L1 immune inhibition in patients with resectable tongue squamous cell carcinoma (TSCC). Int Immunopharmacol. 2018; 59:127–33.
Article20. Jiang P, Zhang Y, Archibald SJ, Wang H. Adoptive cell transfer after chemotherapy enhances survival in patients with resectable HNSCC. Int Immunopharmacol. 2015; 28:208–14.
Article21. Maute RL, Gordon SR, Mayer AT, McCracken MN, Natarajan A, Ring NG, et al. Engineering high-affinity PD-1 variants for optimized immunotherapy and immuno-PET imaging. Proc Natl Acad Sci U S A. 2015; 112:E6506–14.
Article22. Saw PE, Song EW. Phage display screening of therapeutic peptide for cancer targeting and therapy. Protein Cell. 2019; 10:787–807.
Article23. Johnston RJ, Su LJ, Pinckney J, Critton D, Boyer E, Krishnakumar A, et al. VISTA is an acidic pH-selective ligand for PSGL-1. Nature. 2019; 574:565–70.
Article24. Cheng X, Veverka V, Radhakrishnan A, Waters LC, Muskett FW, Morgan SH, et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013; 288:11771–85.
Article25. Zorzi A, Middendorp SJ, Wilbs J, Deyle K, Heinis C. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat Commun. 2017; 8:16092.
Article26. Yao JF, Yang H, Zhao YZ, Xue M. Metabolism of peptide drugs and strategies to improve their metabolic stability. Curr Drug Metab. 2018; 19:892–901.
Article27. Tian Y, Yang D, Ye X, Li Z. Thioether-derived macrocycle for peptide secondary structure fixation. Chem Rec. 2017; 17:874–85.
Article28. Ganesan P, Ramalingam R. Investigation of structural stability and functionality of homodimeric gramicidin towards peptide-based drug: a molecular simulation approach. J Cell Biochem. 2019; 120:4903–11.
Article29. Estrella V, Chen T, Lloyd M, Wojtkowiak J, Cornnell HH, Ibrahim-Hashim A, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013; 73:1524–35.
Article30. Gauthier L, Morel A, Anceriz N, Rossi B, Blanchard-Alvarez A, Grondin G, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019; 177:1701–13.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Peripheral blood immune cell-based biomarkers in anti-PD-1/PD-L1 therapy
- Enhanced Anti-tumor Reactivity of Cytotoxic T Lymphocytes Expressing PD-1 Decoy
- PD-1: A Negative Regulator of Phagocytosis by Tumour-Associated Macrophages in Colon Cancer
- Metformin Suppresses Both PD-L1 Expression in Cancer Cells and Cancer-Induced PD-1 Expression in Immune Cells to Promote Antitumor Immunity
- Immune profiling of mouse lung adenocarcinoma paraffin tissues using multiplex immunofluorescence panel: a pilot study