Immune Netw.
2013 Jun;13(3):86-93. 10.4110/in.2013.13.3.86.
Priming of Autoreactive CD8+ T Cells Is Inhibited by Immunogenic Peptides Which Are Competitive for Major Histocompatibility Complex Class I Binding
- Affiliations
-
- 1Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon 305-701, Korea. ecshin@kaist.ac.kr
- KMID: 2150771
- DOI: http://doi.org/10.4110/in.2013.13.3.86
Abstract
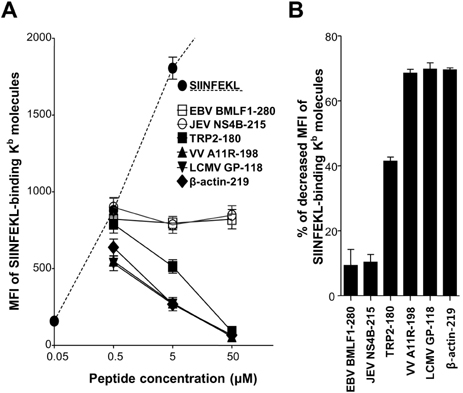
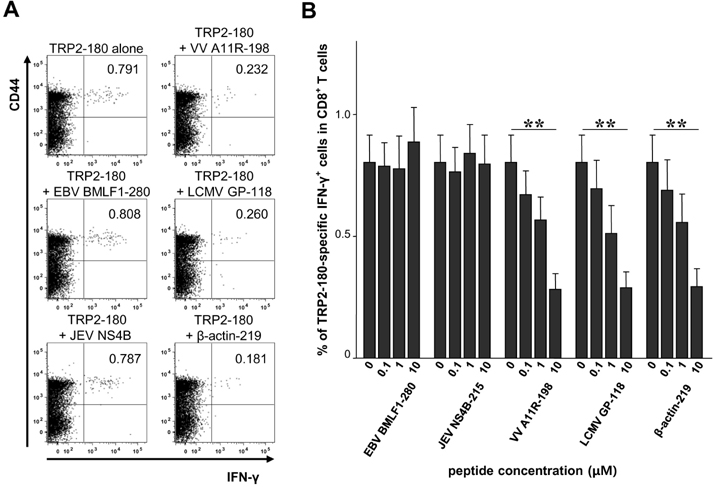
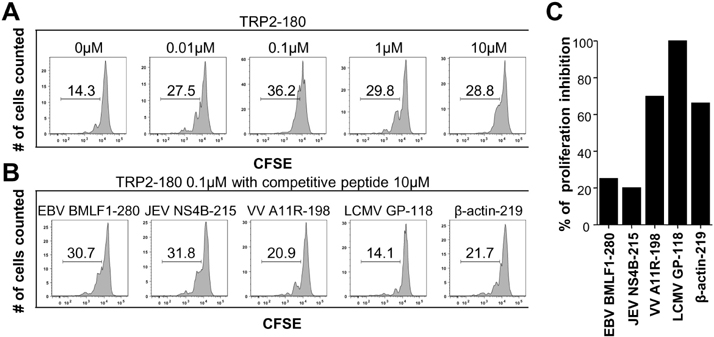
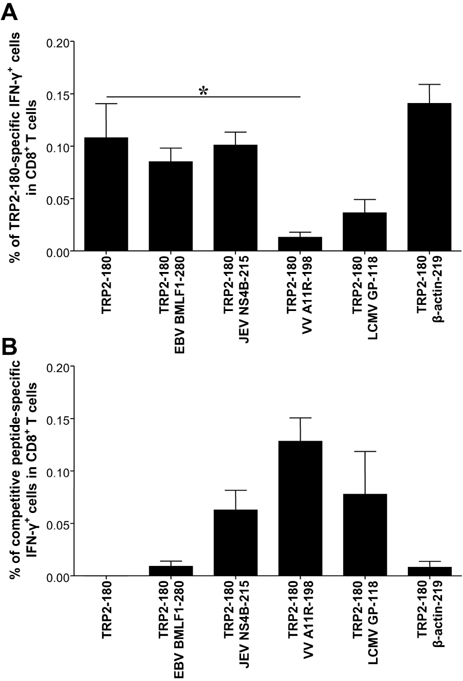
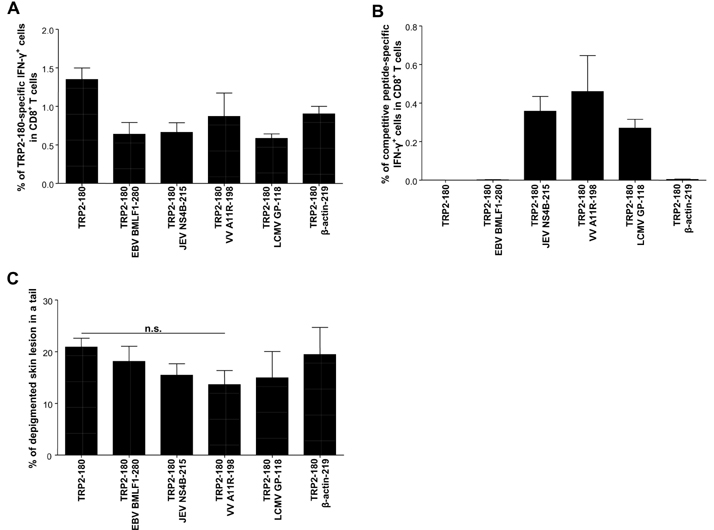
- In the present study, we investigated if priming of autoreactive CD8+ T cells would be inhibited by competitive peptides for major histocompatibility complex (MHC) class I binding. We used a mouse model of vitiligo which is induced by immunization of Kb-binding tyrosinase-related protein 2 (TRP2)-180 peptide. Competitive peptides for Kb binding inhibited IFN-gamma production and proliferation of TRP2-180-specific CD8+ T cells upon ex vivo peptide restimulation, while other MHC class I-binding peptides did not. In mice, the capability of inhibition was influenced by T-cell immunogenicity of the competitive peptides. The competitive peptide with a high T-cell immunogenicity efficiently inhibited priming of TRP2-180-specific CD8+ T cells in vivo, whereas the competitive peptide with a low T-cell immunogenicity did not. Taken together, the inhibition of priming of autoreactive CD8+ T cells depends on not only competition of peptides for MHC class I binding but also competitive peptide-specific CD8+ T cells, suggesting that clonal expansion of autoreactive T cells would be affected by expansion of competitive peptide-specific T cells. This result provides new insights into the development of competitive peptides-based therapy for the treatment of autoimmune diseases.
Keyword
MeSH Terms
Figure
Reference
-
1. Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009; 33:197–207.
Article2. Lee E, Sinha AA. T cell targeted immunotherapy for autoimmune disease. Autoimmunity. 2005; 38:577–596.
Article3. Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987; 329:506–512.
Article4. Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987; 329:512–518.
Article5. Babbitt BP, Matsueda G, Haber E, Unanue ER, Allen PM. Antigenic competition at the level of peptide-Ia binding. Proc Natl Acad Sci U S A. 1986; 83:4509–4513.
Article6. Adorini L, Muller S, Cardinaux F, Lehmann PV, Falcioni F, Nagy ZA. In vivo competition between self peptides and foreign antigens in T-cell activation. Nature. 1988; 334:623–625.
Article7. You S, Cho YH, Byun JS, Shin EC. Melanocyte-specific CD8+ T cells are associated with epidermal depigmentation in a novel mouse model of vitiligo. Clin Exp Immunol. 2013; [Epub ahead of print].
Article8. Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997; 6:715–726.
Article9. Bloom MB, Perry-Lalley D, Robbins PF, Li Y, el-Gamil M, Rosenberg SA, Yang JC. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997; 185:453–459.
Article10. Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005; 201:523–533.
Article11. Karunakaran KP, Rey-Ladino J, Stoynov N, Berg K, Shen C, Jiang X, Gabel BR, Yu H, Foster LJ, Brunham RC. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J Immunol. 2008; 180:2459–2465.
Article12. Trobaugh DW, Yang L, Ennis FA, Green S. Altered effector functions of virus-specific and virus cross-reactive CD8+ T cells in mice immunized with related flaviviruses. Eur J Immunol. 2010; 40:1315–1327.
Article13. Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005; 115:3602–3612.
Article14. Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999; 17:51–88.
Article15. Savir Y, Waysbort N, Antebi YE, Tlusty T, Friedman N. Balancing speed and accuracy of polyclonal T cell activation: a role for extracellular feedback. BMC Syst Biol. 2012; 6:111.
Article16. Curtsinger JM, Lins DC, Mescher MF. CD8+ memory T cells (CD44high, Ly-6C+) are more sensitive than naive cells to (CD44low, Ly-6C-) to TCR/CD8 signaling in response to antigen. J Immunol. 1998; 160:3236–3243.17. Veiga-Fernandes H, Walter U, Bourgeois C, McLean A, Rocha B. Response of naïve and memory CD8+ T cells to antigen stimulation in vivo. Nat Immunol. 2000; 1:47–53.
Article18. Reed C, Katz JM, Hancock K, Balish A, Fry AM. H1N1 Serosurvey Working Group. Prevalence of seropositivity to pandemic influenza A/H1N1 virus in the United States following the 2009 pandemic. PLoS One. 2012; 7:e48187.
Article19. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010; 20:202–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of major histocompatibility complex antigen in Lewis rat cornea
- Relationship between Poor Immunogenicity of HLA-A2-Restricted Peptide Epitopes and Paucity of Naive CD8+ T-Cell Precursors in HLA-A2-Transgenic Mice
- Reconstitution of class I MHC molecules expressed in E. coli and complexed with single antigenic peptides
- Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
- CD43 Expression Regulated by IL-12 Signaling Is Associated with Survival of CD8 T Cells