Yonsei Med J.
2015 Nov;56(6):1522-1529. 10.3349/ymj.2015.56.6.1522.
Effects of Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells on Acute Myocardial Infarction in a Porcine Model
- Affiliations
-
- 1Division of Cardiology, Pusan National University Hospital, Busan, Korea. glaraone@hanmail.net
- 2Division of Thoracic Surgery, Pusan National University Hospital, Busan, Korea.
- 3Division of Pathology, Pusan National University Hospital, Busan, Korea.
- 4Division of Physiology, Pusan National University Hospital, Busan, Korea.
- 5Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2345878
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1522
Abstract
- PURPOSE
Adipose-derived stem cells (ADSCs) are known to be potentially effective in regeneration of damaged tissue. We aimed to assess the effectiveness of intracoronary administration of ADSCs in reducing the infarction area and improving function after acute transmural myocardial infarction (MI) in a porcine model.
MATERIALS AND METHODS
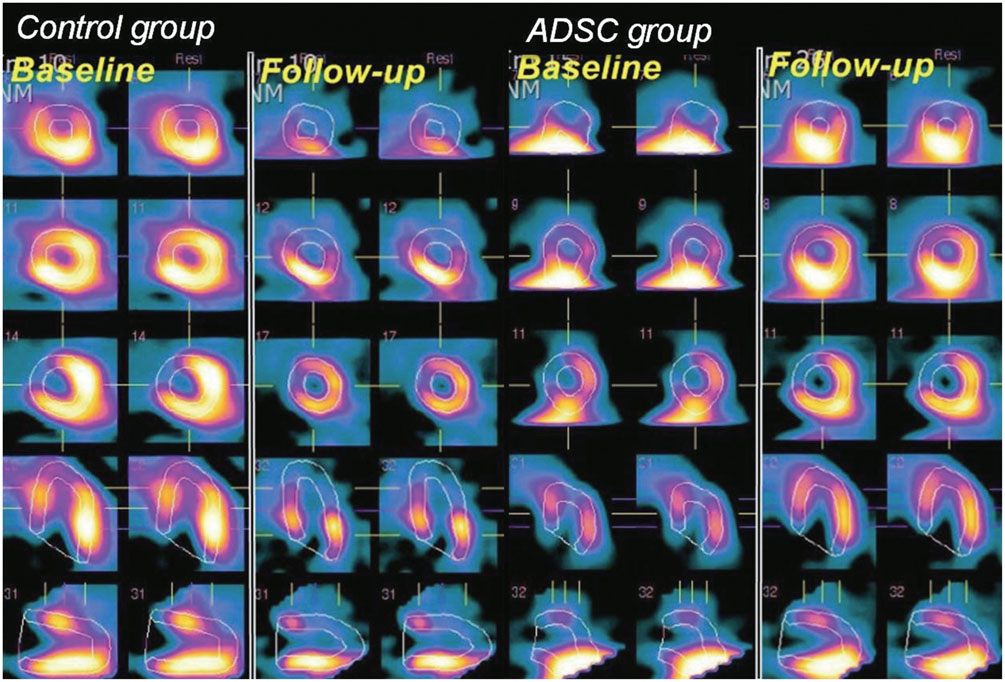
ADSCs were obtained from each pig's abdominal subcutaneous fat tissue by simple liposuction. After 3 passages of 14-days culture, 2 million ADSCs were injected into the coronary artery 30 min after acute transmural MI. At baseline and 4 weeks after the ADSC injection, 99mTc methoxyisobutylisonitrile-single photon emission computed tomography (MIBISPECT) was performed to evaluate the left ventricular volume, left ventricular ejection fraction (LVEF; %), and perfusion defects as well as the myocardial salvage (%) and salvage index. At 4 weeks, each pig was sacrificed, and the heart was extracted and dissected. Gross and microscopic analyses with specific immunohistochemistry staining were then performed.
RESULTS
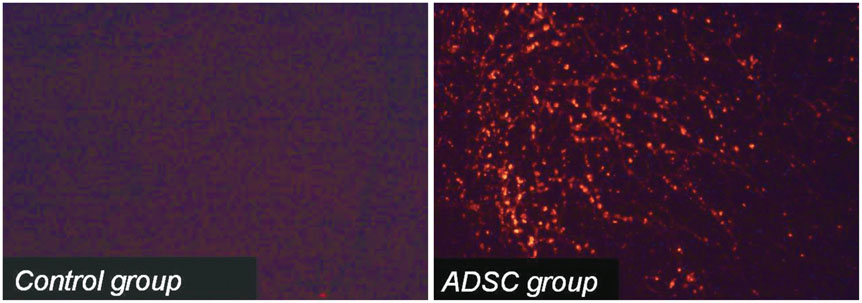
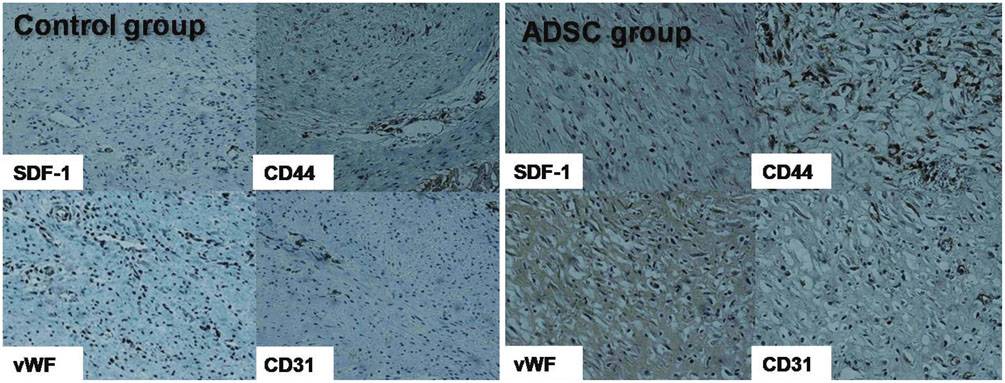
Analysis showed improvement in the perfusion defect, but not in the LVEF in the ADSC group (n=14), compared with the control group (n=14) (perfusion defect, -13.0+/-10.0 vs. -2.6+/-12.0, p=0.019; LVEF, -8.0+/-15.4 vs. -15.9+/-14.8, p=0.181). There was a tendency of reducing left ventricular volume in ADSC group. The ADSCs identified by stromal cell-derived factor-1 (SDF-1) staining were well co-localized by von Willebrand factor and Troponin T staining.
CONCLUSION
Intracoronary injection of cultured ADSCs improved myocardial perfusion in this porcine acute transmural MI model.
Keyword
MeSH Terms
-
Adipose Tissue/cytology
Animals
Bone Marrow Cells/cytology/*metabolism
Chemokine CXCL12
Coronary Vessels
Female
Heart/physiopathology
Heart Ventricles
*Mesenchymal Stromal Cells
Myocardial Infarction/physiopathology/radionuclide imaging/*therapy
*Stem Cell Transplantation
Swine
Technetium Tc 99m Sestamibi/*pharmacology
Tomography, Emission-Computed, Single-Photon/*methods
Troponin T
*Ventricular Function, Left
Chemokine CXCL12
Technetium Tc 99m Sestamibi
Troponin T
Figure
Reference
-
1. Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, et al. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007; 28:2667–2677.
Article2. Cai L, Johnstone BH, Cook TG, Tan J, Fishbein MC, Chen PS, et al. IFATS collection: human adipose tissue-derived stem cells induce angiogenesis and nerve sprouting following myocardial infarction, in conjunction with potent preservation of cardiac function. Stem Cells. 2009; 27:230–237.
Article3. Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010; 31:489–501.
Article4. Houtgraaf JH, den Dekker WK, van Dalen BM, Springeling T, de Jong R, van Geuns RJ, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012; 59:539–540.
Article5. Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004; 14:311–324.
Article6. Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006; 17:279–290.
Article7. Cho HH, Kim YJ, Kim JT, Song JS, Shin KK, Bae YC, et al. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell Physiol Biochem. 2009; 24:511–518.
Article8. Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008; 103:1204–1219.
Article9. Miranville A, Heeschen C, Sengenès C, Curat CA, Busse R, Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004; 110:349–355.
Article10. Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004; 109:656–663.
Article11. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.
Article12. Wang JS, Shum-Tim D, Galipeau J, Chedrawy E, Eliopoulos N, Chiu RC. Marrow stromal cells for cellular cardiomyoplasty: feasibility and potential clinical advantages. J Thorac Cardiovasc Surg. 2000; 120:999–1005.
Article13. Wakitani S, Saito T, Caplan AI. Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve. 1995; 18:1417–1426.
Article14. Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004; 109:1292–1298.
Article15. Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009; 29:1723–1729.16. Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001; 7:211–228.
Article17. Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, et al. Human mesenchymal stem cells engraft and demonstrate sitespecific differentiation after in utero transplantation in sheep. Nat Med. 2000; 6:1282–1286.
Article18. Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001; 410:701–705.
Article19. Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, et al. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005; 96:127–137.
Article20. Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: the scientific foundations of cardiac repair. J Clin Invest. 2005; 115:572–583.
Article21. Kuethe F, Richartz BM, Sayer HG, Kasper C, Werner GS, Höffken K, et al. Lack of regeneration of myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans with large anterior myocardial infarctions. Int J Cardiol. 2004; 97:123–127.
Article22. Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005; 111:150–156.
Article23. Yang D, Wang W, Li L, Peng Y, Chen P, Huang H, et al. The relative contribution of paracine effect versus direct differentiation on adipose-derived stem cell transplantation mediated cardiac repair. PLoS One. 2013; 8:e59020.
Article24. Moelker AD, Baks T, Wever KM, Spitskovsky D, Wielopolski PA, van Beusekom HM, et al. Intracoronary delivery of umbilical cord blood derived unrestricted somatic stem cells is not suitable to improve LV function after myocardial infarction in swine. J Mol Cell Cardiol. 2007; 42:735–745.
Article25. Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004; 363:783–784.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration
- Intramyocardial Injection of Stem Cells in Pig Myocardial Infarction Model: The First Trial in Korea
- Adipose-derived stem cells: characterization and clinical application
- Fat grafts enriched with adipose-derived stem cells
- A Randomized, Open-Label, Multicenter Trial for the Safety and Efficacy of Adult Mesenchymal Stem Cells after Acute Myocardial Infarction