Ann Dermatol.
2016 Aug;28(4):457-463. 10.5021/ad.2016.28.4.457.
Expression of Epidermal c-Kit+ of Vitiligo Lesions Is Related to Responses to Excimer Laser
- Affiliations
-
- 1Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csesnumd@gmail.com
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2344816
- DOI: http://doi.org/10.5021/ad.2016.28.4.457
Abstract
- BACKGROUND
The survival and growth of melanocytes are controlled by the binding of stem cell factor to its cell surface receptor c-kit+ (CD117). We have observed that c-kit+ melanocytes existed in some lesions of vitiligo, while Melan A+ cells were absent.
OBJECTIVE
To verify possible relation between c-kit+ expression and treatment response in non-segmental vitiligo lesions.
METHODS
Skin biopsies were done from the center of the 47 lesions from the 47 patients with non-segmental vitiligo. Expression of c-kit+ and Melan A, and amounts of melanin in the epidermis were assessed in each lesion, and treatment responses to excimer laser were evaluated.
RESULTS
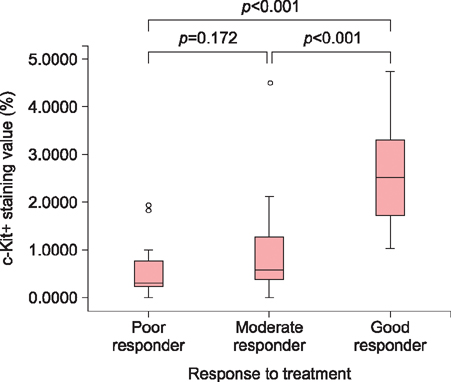
Thirty-five of the 47 lesions (74.5%) had c-kit+ phenotypes. There was significant difference of c-kit staining value between good responders in 3 months of excimer laser treatment (average of 24 sessions) and the others.
CONCLUSION
c-Kit expression in vitiliginous epidermis may be related to better treatment responses to excimer laser.
Keyword
MeSH Terms
Figure
Reference
-
1. Bolognia J, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Edinburgh: Mosby Elsevier;2008.2. Nicolaidou E, Antoniou C, Miniati A, Lagogianni E, Matekovits A, Stratigos A, et al. Childhood- and later-onset vitiligo have diverse epidemiologic and clinical characteristics. J Am Acad Dermatol. 2012; 66:954–958.
Article3. Agarwal S, Tamta M. Clinical predictors for the course of nonsegmental vitiligo. Clin Exp Dermatol. 2013; 38:669–670.
Article4. Singh ZN, Tretiakova MS, Shea CR, Petronic-Rosic VM. Decreased CD117 expression in hypopigmented mycosis fungoides correlates with hypomelanosis: lessons learned from vitiligo. Mod Pathol. 2006; 19:1255–1260.
Article5. El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, McCalmont TH. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002; 26:450–457.6. Steitz J, Wenzel J, Gaffal E, Tüting T. Initiation and regulation of CD8+T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004; 83:797–803.
Article7. Mandelcorn-Monson RL, Shear NH, Yau E, Sambhara S, Barber BH, Spaner D, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003; 121:550–556.
Article8. Kasamatsu S, Hachiya A, Higuchi K, Ohuchi A, Kitahara T, Boissy RE. Production of the soluble form of KIT, s-KIT, abolishes stem cell factor-induced melanogenesis in human melanocytes. J Invest Dermatol. 2008; 128:1763–1772.
Article9. Richards KA, Fukai K, Oiso N, Paller AS. A novel KIT mutation results in piebaldism with progressive depigmentation. J Am Acad Dermatol. 2001; 44:288–292.
Article10. Kim YJ, Lee JB, Kim SJ, Lee SC, Won YH, Yun SJ. Amelanotic acral melanoma associated with KIT mutation and vitiligo. Ann Dermatol. 2015; 27:201–205.
Article11. Aoki H, Hara A, Motohashi T, Kunisada T. Protective effect of Kit signaling for melanocyte stem cells against radiation-induced genotoxic stress. J Invest Dermatol. 2011; 131:1906–1915.
Article12. Do JE, Shin JY, Kim DY, Hann SK, Oh SH. The effect of 308nm excimer laser on segmental vitiligo: a retrospective study of 80 patients with segmental vitiligo. Photodermatol Photoimmunol Photomed. 2011; 27:147–151.
Article13. Nicolaidou E, Antoniou C, Stratigos A, Katsambas AD. Narrowband ultraviolet B phototherapy and 308-nm excimer laser in the treatment of vitiligo: a review. J Am Acad Dermatol. 2009; 60:470–477.
Article14. Roskoski R Jr. Signaling by Kit protein-tyrosine kinase--the stem cell factor receptor. Biochem Biophys Res Commun. 2005; 337:1–13.
Article15. Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, Kobayashi T, et al. Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol. 2004; 202:463–475.
Article16. Mizutani Y, Hayashi N, Kawashima M, Imokawa G. A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch Dermatol Res. 2010; 302:283–294.
Article17. Kawalek AZ, Spencer JM, Phelps RG. Combined excimer laser and topical tacrolimus for the treatment of vitiligo: a pilot study. Dermatol Surg. 2004; 30:130–135.
Article18. Katayama I, Ashida M, Maeda A, Eishi K, Murota H, Bae SJ. Open trial of topical tacalcitol [1 alpha 24(OH)2D3] and solar irradiation for vitiligo vulgaris: upregulation of c-Kit mRNA by cultured melanocytes. Eur J Dermatol. 2003; 13:372–376.19. Jeon S, Kim NH, Kim JY, Lee AY. Stem cell factor induces ERM proteins phosphorylation through PI3K activation to mediate melanocyte proliferation and migration. Pigment Cell Melanoma Res. 2009; 22:77–85.
Article20. Lennartsson J, Blume-Jensen P, Hermanson M, Pontén E, Carlberg M, Rönnstrand L. Phosphorylation of Shc by Src family kinases is necessary for stem cell factor receptor/c-kit mediated activation of the Ras/MAP kinase pathway and c-fos induction. Oncogene. 1999; 18:5546–5553.
Article21. Kaliyadan F, Ashique KT. Optimizing the efficacy of targeted phototherapy by marking early vitiligo lesions after visualizing under a Wood's lamp. J Am Acad Dermatol. 2014; 71:e69.
Article22. Norris A, Todd C, Graham A, Quinn AG, Thody AJ. The expression of the c-kit receptor by epidermal melanocytes may be reduced in vitiligo. Br J Dermatol. 1996; 134:299–306.
Article23. Tachibana M, Takeda K, Nobukuni Y, Urabe K, Long JE, Meyers KA, et al. Ectopic expression of MITF, a gene for Waardenburg syndrome type 2, converts fibroblasts to cells with melanocyte characteristics. Nat Genet. 1996; 14:50–54.
Article24. Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature. 1998; 391:298–301.
Article25. van den Boorn JG, Konijnenberg D, Dellemijn TA, van den Boorn JG, Bos JD, Melief CJ, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009; 129:2220–2232.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Effects of 308 nm Excimer Laser in the Treatment of Vitiligo Patients: A Single Center Study in Korea
- Therapeutic Effects of 308 nm Excimer Laser in the Treatment of Vitiligo on the Head and Neck Area
- Short-term Effects of 308-nm Xenon-chloride Excimer Laser and Narrow-band Ultraviolet B in the Treatment of Vitiligo: A Comparative Study
- A Case of Refractory Vitiligo That Was Treated with a Combination of Non-ablative 1550-nm Erbium:Glass Fractional Laser, Narrow-band UVB, and a Topical Agent
- Treatment of Laser Therapy-Induced Punctate Leukoderma Using a 308-nm Excimer Laser