J Korean Soc Radiol.
2016 Aug;75(2):113-120. 10.3348/jksr.2015.73.6.113.
Diffusion Tensor Tractography of the Brainstem Pyramidal Tract: A Study on the Optimal Reduction Factor in Parallel Imaging
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. jaehkim@snu.ac.kr
- KMID: 2344800
- DOI: http://doi.org/10.3348/jksr.2015.73.6.113
Abstract
- PURPOSE
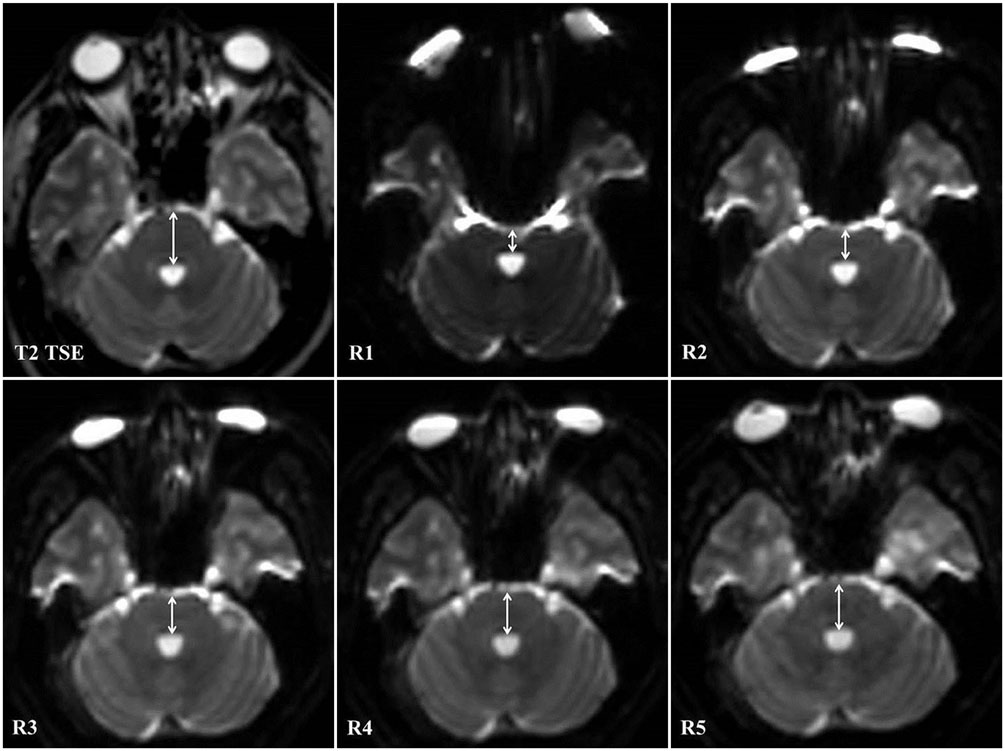
Parallel imaging mitigates susceptibility artifacts that can adversely affect diffusion tensor tractography (DTT) of the pons depending on the reduction (R) factor. We aimed to find the optimal R factor for DTT of the pons that would allow us to visualize the largest possible number of pyramidal tract fibers.
MATERIALS AND METHODS
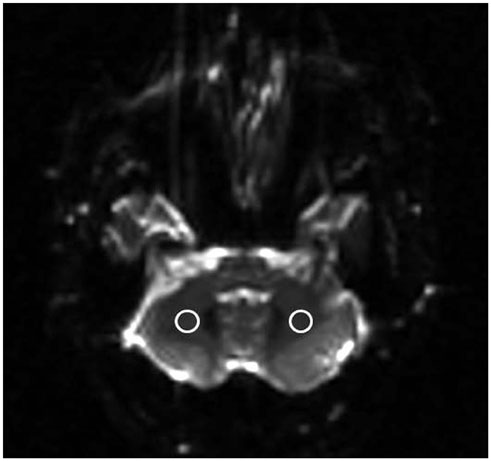
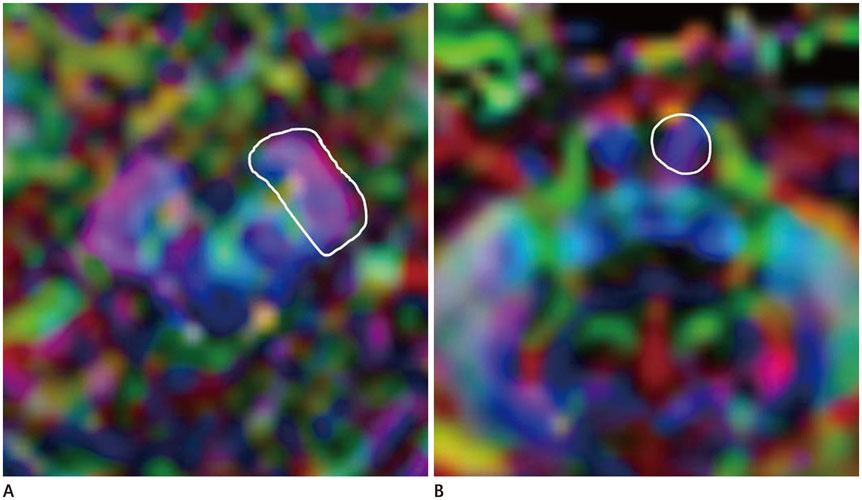
Diffusion tensor imaging was performed on 10 healthy subjects at 3 Tesla based on single-shot echo-planar imaging using the following parameters: b value, 1000 s/mm²; gradient direction, 15; voxel size, 2 × 2 × 2 mm³; and R factors, 1, 2, 3, 4, and 5. DTT of the right and left pyramidal tracts in the pons was conducted in all subjects. Signal-to-noise ratio (SNR), image distortion, and the number of fibers in the tracts were compared across R factors.
RESULTS
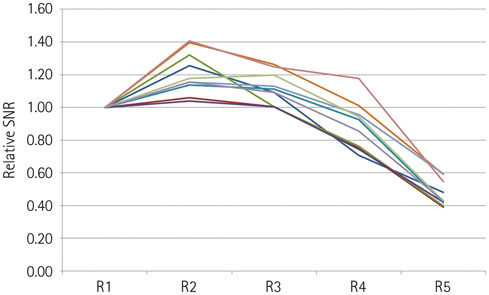
SNR, image distortion, and fiber number were significantly different according to R factor. Maximal SNR was achieved with an R factor of 2. Image distortion was minimal with an R factor of 5. The number of visible fibers was greatest with an R factor of 3.
CONCLUSION
R factor 3 is optimal for DTT of the pontine pyramidal tract. A balanced consideration of SNR and image distortion, which do not have the same dependence on the R factor, is necessary for DTT of the pons.
MeSH Terms
Figure
Reference
-
1. Noël P, Bammer R, Reinhold C, Haider MA. Parallel imaging artifacts in body magnetic resonance imaging. Can Assoc Radiol J. 2009; 60:91–98.2. Glockner JF, Hu HH, Stanley DW, Angelos L, King K. Parallel MR imaging: a user's guide. Radiographics. 2005; 25:1279–1297.3. Bhagat YA, Emery DJ, Naik S, Yeo T, Beaulieu C. Comparison of generalized autocalibrating partially parallel acquisitions and modified sensitivity encoding for diffusion tensor imaging. AJNR Am J Neuroradiol. 2007; 28:293–298.4. Wilson M, Tench CR, Morgan PS, Blumhardt LD. Pyramidal tract mapping by diffusion tensor magnetic resonance imaging in multiple sclerosis: improving correlations with disability. J Neurol Neurosurg Psychiatry. 2003; 74:203–207.5. Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007; 245:367–384.6. Rollins NK. Clinical applications of diffusion tensor imaging and tractography in children. Pediatr Radiol. 2007; 37:769–780.7. Jaermann T, Crelier G, Pruessmann KP, Golay X, Netsch T, van Muiswinkel AM, et al. SENSE-DTI at 3 T. Magn Reson Med. 2004; 51:230–236.8. Jaermann T, Pruessmann KP, Valavanis A, Kollias S, Boesiger P. Influence of SENSE on image properties in high-resolution single-shot echo-planar DTI. Magn Reson Med. 2006; 55:335–342.9. Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold MA, Jakob PM. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Top Magn Reson Imaging. 2004; 15:223–236.10. Morelli JN, Runge VM, Feiweier T, Kirsch JE, Williams KW, Attenberger UI. Evaluation of a modified Stejskal-Tanner diffusion encoding scheme, permitting a marked reduction in TE, in diffusion-weighted imaging of stroke patients at 3 T. Invest Radiol. 2010; 45:29–35.11. Nagae-Poetscher LM, Jiang H, Wakana S, Golay X, van Zijl PC, Mori S. High-resolution diffusion tensor imaging of the brain stem at 3 T. AJNR Am J Neuroradiol. 2004; 25:1325–1330.12. Lui YW, Law M, Chacko-Mathew J, Babb JS, Tuvia K, Allen JC, et al. Brainstem corticospinal tract diffusion tensor imaging in patients with primary posterior fossa neoplasms stratified by tumor type: a study of association with motor weakness and outcome. Neurosurgery. 2007; 61:1199–1207.13. Giussani C, Poliakov A, Ferri RT, Plawner LL, Browd SR, Shaw DW, et al. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. Neuroimage. 2010; 52:217–223.14. Prabhu SP, Ng S, Vajapeyam S, Kieran MW, Pollack IF, Geyer R, et al. DTI assessment of the brainstem white matter tracts in pediatric BSG before and after therapy: a report from the Pediatric Brain Tumor Consortium. Childs Nerv Syst. 2011; 27:11–18.15. Khatua S, Hou P, Bodiwala R, Wolff J, Hamilton J, Patil S, et al. Preliminary experience with diffusion tensor imaging before and after re-irradiation treatments in children with progressive diffuse pontine glioma. Childs Nerv Syst. 2014; 30:925–930.16. Gudbjartsson H, Patz S. The Rician distribution of noisy MRI data. Magn Reson Med. 1995; 34:910–914.17. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999; 45:265–269.18. Ulrich NH, Kockro RA, Bellut D, Amaxopoulou C, Bozinov O, Burkhardt JK, et al. Brainstem cavernoma surgery with the support of pre- and postoperative diffusion tensor imaging: initial experiences and clinical course of 23 patients. Neurosurg Rev. 2014; 37:481–491.19. Merhof D, Soza G, Stadlbauer A, Greiner G, Nimsky C. Correction of susceptibility artifacts in diffusion tensor data using non-linear registration. Med Image Anal. 2007; 11:588–603.20. Bammer R, Auer M, Keeling SL, Augustin M, Stables LA, Prokesch RW, et al. Diffusion tensor imaging using singleshot SENSE-EPI. Magn Reson Med. 2002; 48:128–136.21. Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging. 2012; 36:55–72.22. Lee JS, Han MK, Kim SH, Kwon OK, Kim JH. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: topographical correlation with clinical symptoms. Neuroimage. 2005; 26:771–776.23. Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, et al. Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol. 2006; 27:2168–2178.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications
- Mini-Review of Studies Reporting the Repeatability and Reproducibility of Diffusion Tensor Imaging
- Diffusion Tensor Tractography in Two Cases of Kernohan-Woltman Notch Phenomenon
- Disruption of the Corticoreticular Tract in Pediatric Patients With Trunk Instability: A Diffusion Tensor Tractography Study
- Clinical Usefulness of Diffusion Tensor Image Tractography in Stroke Patients: Report of two cases