J Korean Ophthalmol Soc.
2014 Jan;55(1):13-19.
The Efficacy of Low-Dose Systemic Cyclosporine for Graft Failure after Penetrating Keratoplasty in High-Risk Group
- Affiliations
-
- 1Institute of Vision Research, Department of Ophthalmology, Yonsei University College of Medicine, Seoul, Korea. shadik@yuhs.ac
- 2Institute of Corneal Dystrophy Research, Yonsei University College of Medicine, Seoul, Korea.
- 3Severance Institute for Vascular and Metabolic Research, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
To investigate the effect of low-dose systemic cyclosporine A (CsA) in preventing graft failure after high-risk penetrating keratoplasty (PKP).
METHODS
In this retrospective study, 36 eyes of 25 patients who underwent PKP were evaluated. At 24 months postoperatively, the failure rate in the CsA group (n = 19) was compared to the control group (n = 17). For subgroup analysis, the failure rate in the CsA group (n = 9) and control group (n = 9) was compared in patients who underwent a repeat PKP. The patients' side-effect profile was also collected.
RESULTS
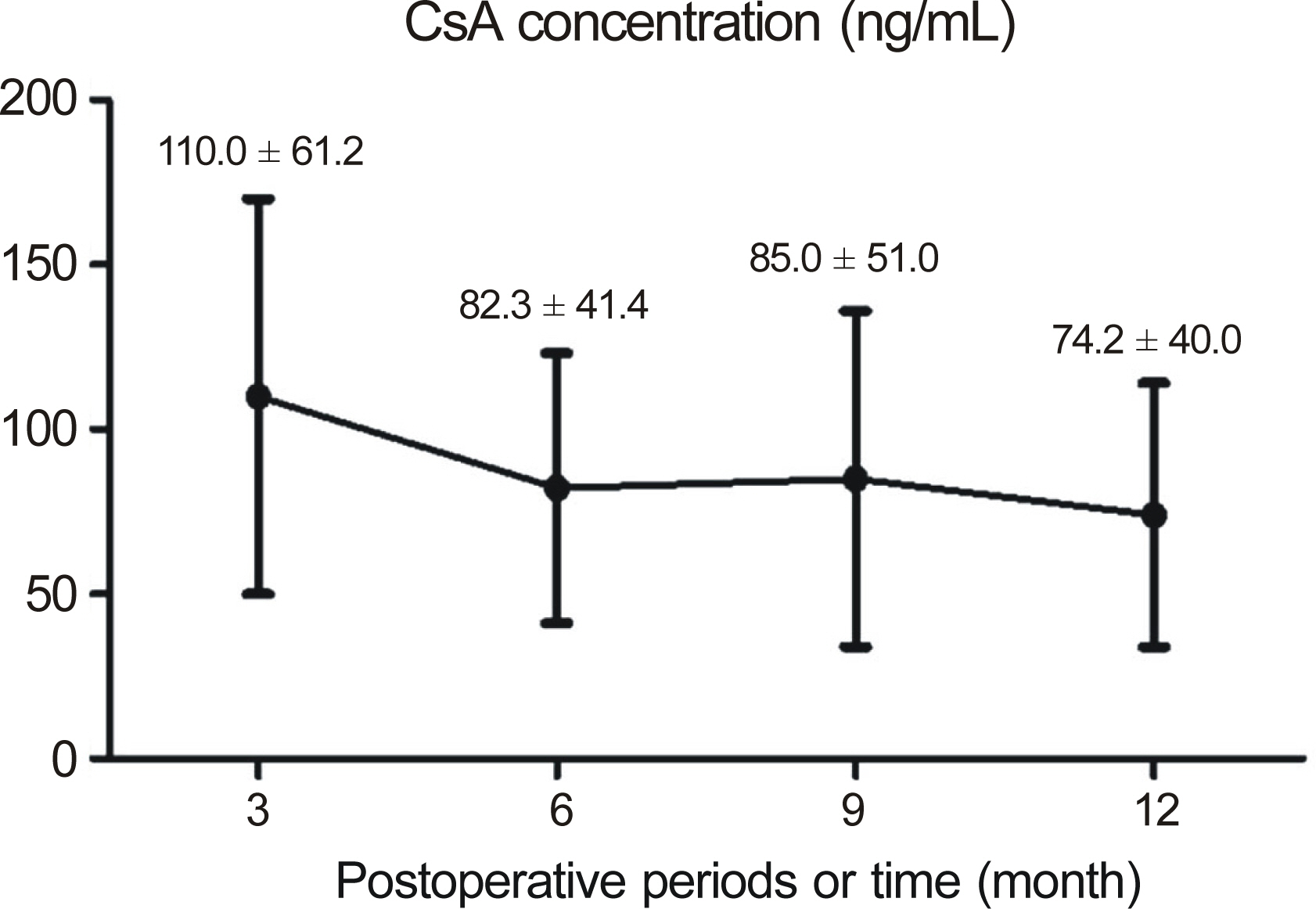
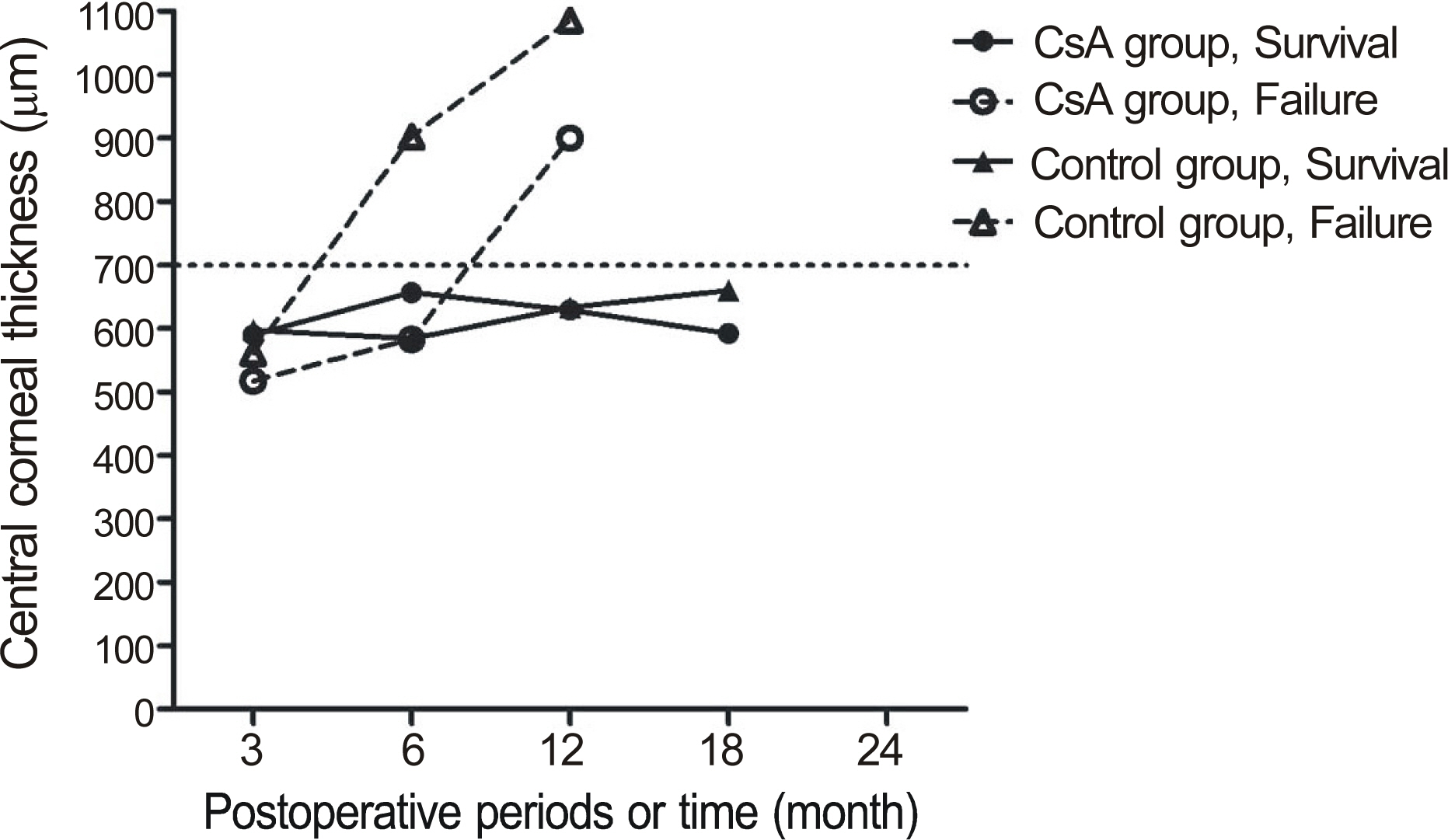
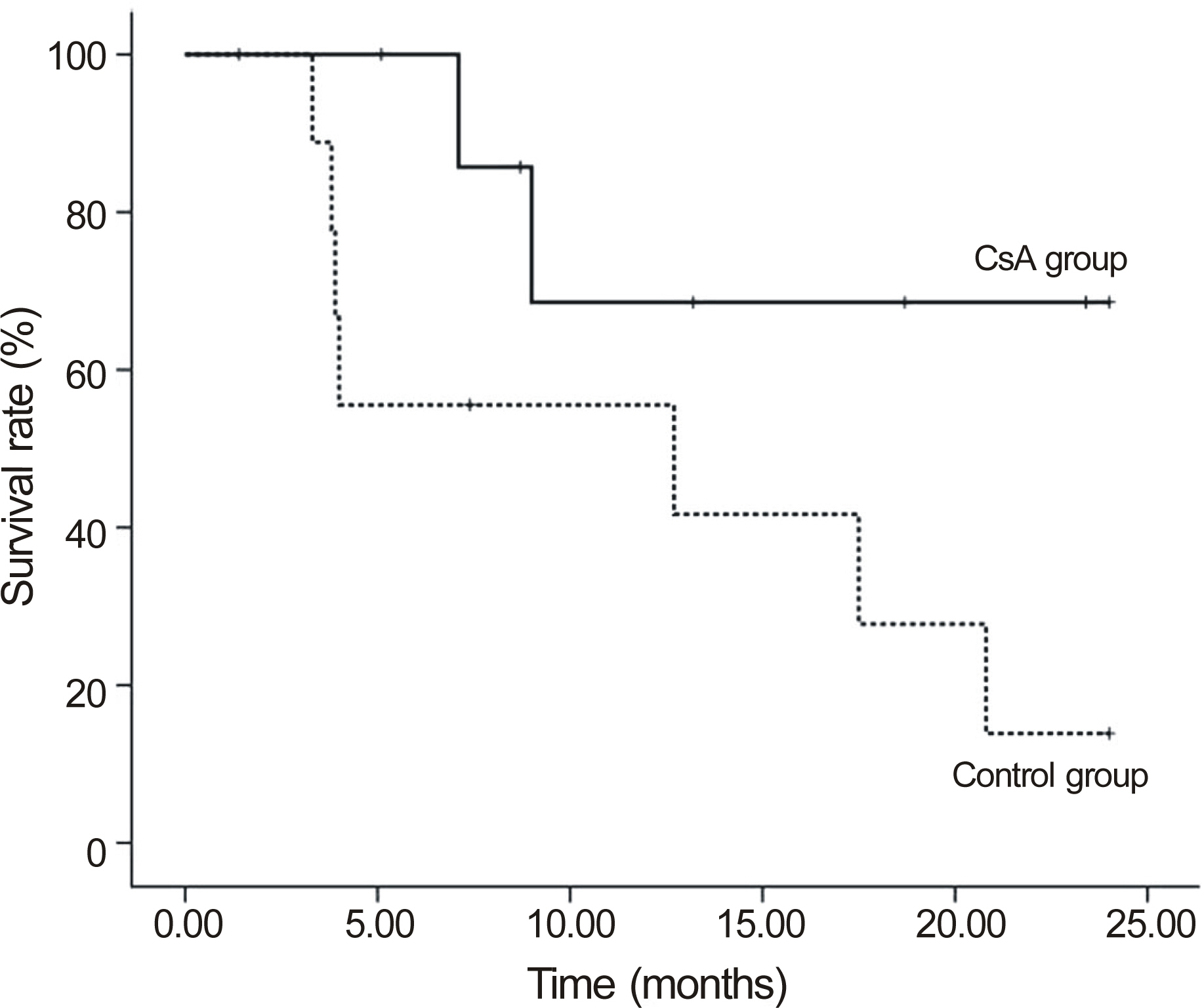
The median follow-up in the CsA group was 32.8 months and 28.9 months in the control group. Graft failure occurred in 31.6% CsA patients and in 68.4% control patients (p = 0.311). In patients with repeat PKP, the failure rate in the CsA group was significantly lower than the control group (22.2% vs. 77.8%, p = 0.018). In one case (5.26%), CsA was discontinued due to gastroinstestinal discomfort.
CONCLUSIONS
Low-dose CsA was not beneficial compared to conventional therapy in high-risk PKP patients. However, in the repeat PKP subgroup, the incidence of graft failure was lower with low-dose CsA than with conventional therapy. Although further study is necessary, adding low-dose CsA might be beneficial for repeat PKP patients.
MeSH Terms
Figure
Reference
-
References
1. Luengo-Gimeno F, Tan DT, Mehta JS.Evolution of deep anterior lamellar keratoplasty (DALK). Ocul Surf. 2011; 9:98–110.
Article2. Lee WB, Jacobs DS, Musch DC. . Descemet's stripping endo-thelial keratoplasty: safety and outcomes: a report by the American Academy of Ophthalmology. Ophthalmology. 2009; 116:1818–30.3. Keenan TD, Carley F, Yeates D. . Trends in corneal graft sur-gery in the UK. Br J Ophthalmol. 2011; 95:468–72.
Article4. Williams KA, Lowe M, Bartlett C. . Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008; 86:1720–4.
Article5. Williams KA, Roder D, Esterman A. . Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology. 1992; 99:403–14.6. Tan DT, Janardhanan P, Zhou H. . Penetrating keratoplasty in Asian eyes: the Singapore Corneal Transplant Study. Ophthalmology. 2008; 115:975–82.e1.7. Thompson RW Jr, Price MO, Bowers PJ, Price FW Jr.Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003; 110:1396–402.
Article8. Wagoner MD, Ba-Abbad R, Sutphin JE, Zimmerman MB.Corneal transplant survival after onset of severe endothelial rejection. Ophthalmology. 2007; 114:1630–6.
Article9. Joseph A, Raj D, Shanmuganathan V. . Tacrolimus im-munosuppression in high-risk corneal grafts. Br J Ophthalmol. 2007; 91:51–5.
Article10. Chatel MA, Larkin DF.Sirolimus and mycophenolate as combina-tion prophylaxis in corneal transplant recipients at high rejection risk. Am J Ophthalmol. 2010; 150:179–84.
Article11. Birnbaum F, Mayweg S, Reis A. . Mycophenolate mofetil (MMF) following penetrating high-risk keratoplasty: long-term re-sults of a prospective, randomised, multicentre study. Eye (Lond). 2009; 23:2063–70.
Article12. Calne RY, White DJ, Thiru S. . Cyclosporin A in patients re-ceiving renal allografts from cadaver donors. Lancet. 1978; 2:1323–7.
Article13. Starzl TE, Klintmalm GB, Porter KA. . Liver transplantation with use of cyclosporin a and prednisone. N Engl J Med. 1981; 305:266–9.
Article14. Borghi A, Corazza M, Mantovani L. . Prolonged cyclosporine treatment of severe or recalcitrant psoriasis: descriptive study in a series of 20 patients. Int J Dermatol. 2012; 51:1512–6.
Article15. Lichtiger S, Present DH, Kornbluth A. . Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994; 330:1841–5.
Article16. Poon AC, Forbes JE, Dart JK. . Systemic cyclosporin A in high risk penetrating keratoplasties: a case-control study. Br J Ophthalmol. 2001; 85:1464–9.
Article17. Reinhard T, Sundmacher R, Heering P.Systemic ciclosporin A in high-risk keratoplasties. Graefes Arch Clin Exp Ophthalmol. 1996; 234(Suppl 1):S115–21.
Article18. Hill JC.The use of systemic cyclosporin a in human corneal trans-plantation: a preliminary report. Doc Ophthalmol. 1986; 62:337–44.
Article19. Hill JC.Systemic cyclosporine in high-risk keratoplasty. Short-versus long-term therapy. Ophthalmology. 1994; 101:128–33.20. Reinhard T, Sundmacher R, Godehardt E, Heering P.Preventive systemic cyclosporin A after keratoplasty at increased risk for immune reactions as the only elevated risk factor. Ophthalmologe. 1997; 94:496–500.21. Shimazaki J, Den S, Omoto M. . Prospective, randomized study of the efficacy of systemic cyclosporine in high-risk corneal transplantation. Am J Ophthalmol. 2011; 152:33–9.e1.
Article22. Robert PY, Delbosc B, Preux PM. . [Treatment with cyclo-sporin A, with low doses, in high-risk penetrating keratoplasties. A bi-centric study of 90 cases]. J Fr Ophtalmol. 1997; 20:507–14.23. Boisjoly HM, Tourigny R, Bazin R. . Risk factors of corneal graft failure. Ophthalmology. 1993; 100:1728–35.
Article24. Kim EC, Kim MS.Graft Rejection in Penetrating Keratoplasty. J Korean Ophthalmol Soc. 2003; 44:289–95.25. Weisbrod DJ, Sit M, Naor J, Slomovic AR.Outcomes of repeat penetrating keratoplasty and risk factors for graft failure. Cornea. 2003; 22:429–34.
Article26. Woods JE, Anderson CF, DeWeerd JH. . High-dosage intra-venously administered methylprednisolone in renal transplantation. A preliminary report. JAMA. 1973; 223:896–9.
Article27. Graham RM.Cyclosporine: mechanisms of action and toxicity. Cleve Clin J Med. 1994; 61:308–13.28. Algros MP, Angonin R, Delbosc B. . Danger of systemic cyclo-sporine for corneal graft. Cornea. 2002; 21:613–4.
Article29. Patel J, Kobashigawa JA.Minimization of immunosuppression: transplant immunology. Transpl Immunol. 2008; 20(1-2):48–54.30. Reinhard T, Reis A, Böhringer D. . Systemic mycophenolate mofetil in comparison with systemic cyclosporin A in high-risk keratoplasty patients: 3 years' results of a randomized prospective clinical trial. Graefes Arch Clin Exp Ophthalmol. 2001; 239:367–72.
Article31. Rumelt S, Bersudsky V, Blum-Hareuveni T, Rehany U.Systemic cyclosporin A in high failure risk, repeated corneal transplantation. Br J Ophthalmol. 2002; 86:988–92.32. Völker-Dieben HJ, D'Amaro J, Kok-van Alphen CC. Hierarchy of prognostic factors for corneal allograft survival. Aust N Z J Ophthalmol. 1987; 15:11–8.33. Fasolo A, Capuzzo C, Fornea M. . Risk factors for graft failure after penetrating keratoplasty: 5-year follow-up from the corneal transplant epidemiological study. Cornea. 2011; 30:1328–35.
Article34. Anshu A, Lim LS, Htoon HM, Tan DT.Postoperative risk factors influencing corneal graft survival in the Singapore Corneal Transplant Study. Am J Ophthalmol. 2011; 151:442–8.e1.
Article35. Keown P, Kahan BD, Johnston A. . Optimization of cyclo-sporine therapy with new therapeutic drug monitoring strategies: report from the International Neoral TDM Advisory Consensus Meeting (Vancouver, November 1997). Transplant Proc. 1998; 30:1645–9.
Article36. Pescovitz MD, Barbeito R. Simulect US01 Study Group. Two-hour post-dose cyclosporine level is a better predictor than trough level of acute rejection of renal allografts. Clin Transplant. 2002; 16:378–82.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cyclosporin A in High Risk Penetrating Keratoplasty
- Risk Factors of Graft Failure in Post-Keratoplasty Ocular Hypertension
- A Clinical Study of Risk Factors of Graft Rejection for Penetrating Keratoplasty
- Clinical Results of Graft Rejection Related to Graft Size after Keratoplasty in Keratoconus
- Effect of Donor Age on Graft Survival in Primary Penetrating Keratoplasty with Imported Donor Corneas