J Korean Ophthalmol Soc.
2012 Feb;53(2):323-332.
The Antifibrotic Effects of alpha-Tocotrienols in Primary Cultured Orbital Fibroblasts from Thyroid-Associated Ophthalmopathy Patients
- Affiliations
-
- 1Department of Ophthalmology, Inha University School of Medicine, Incheon, Korea. ksm0724@inha.ac.kr
Abstract
- PURPOSE
To evaluate the antiproliferative and antifibrotic effects of alpha-tocotrienols in primary cultured orbital fibroblasts from thyroid-associated ophthalmopathy (TAO) patients.
METHODS
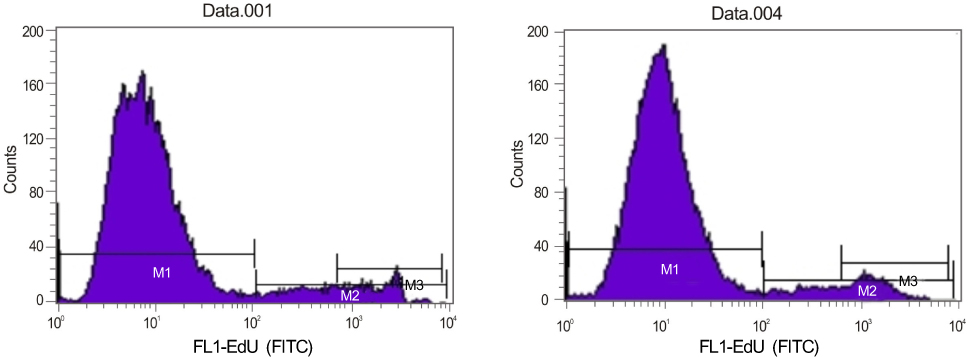
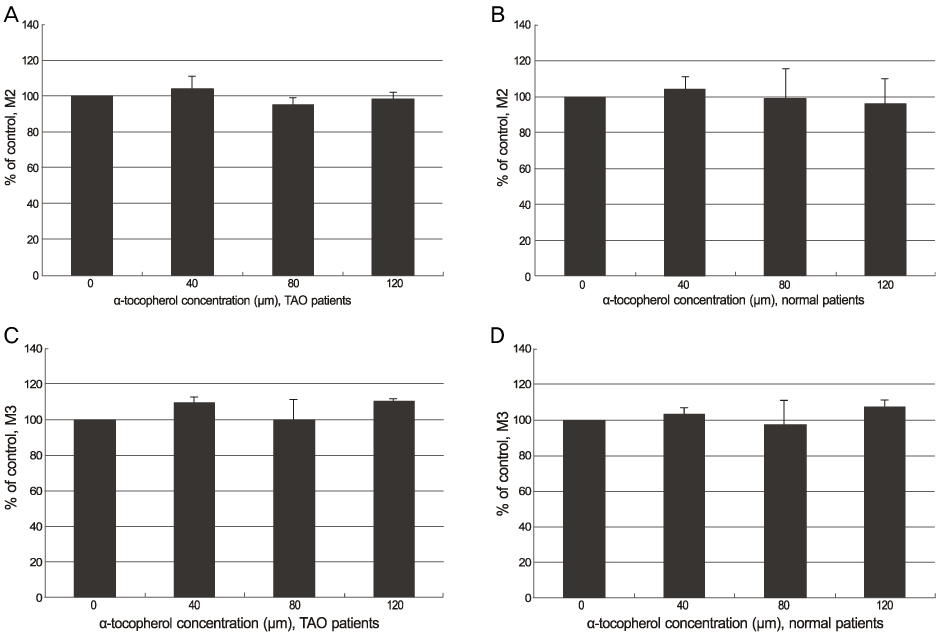
Orbital fibroblasts were cultured (5 eyes from TAO patients, 5 eyes from normal patients) and classified into a control group, alpha-tocotrienol group and alpha-tocopherol group. The cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The proliferation of orbital fibroblasts was measured using the Click-iT(TM) assay. The collagen production of the control and alpha-tocotrienol groups was measured using a hydroxyproline assay.
RESULTS
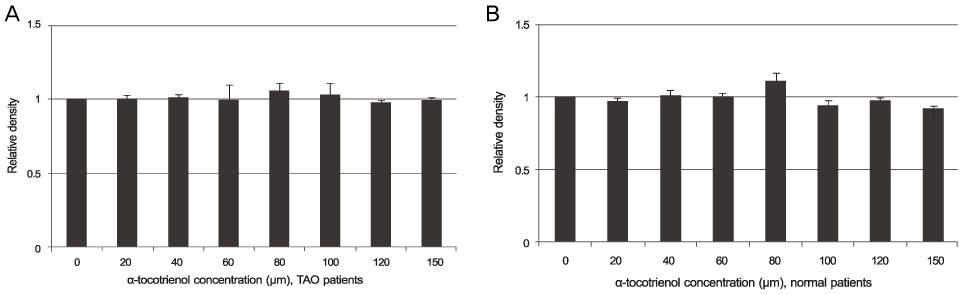
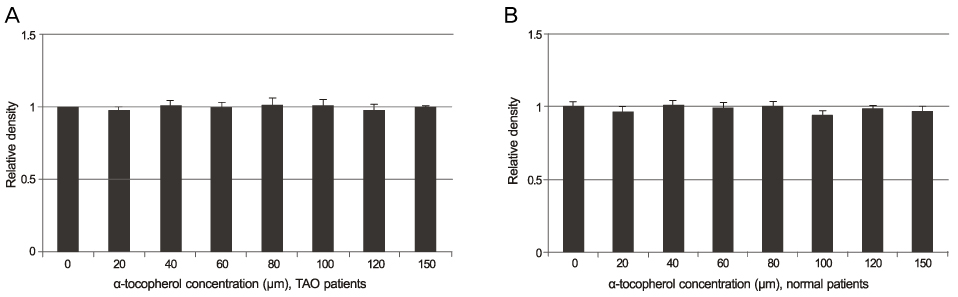
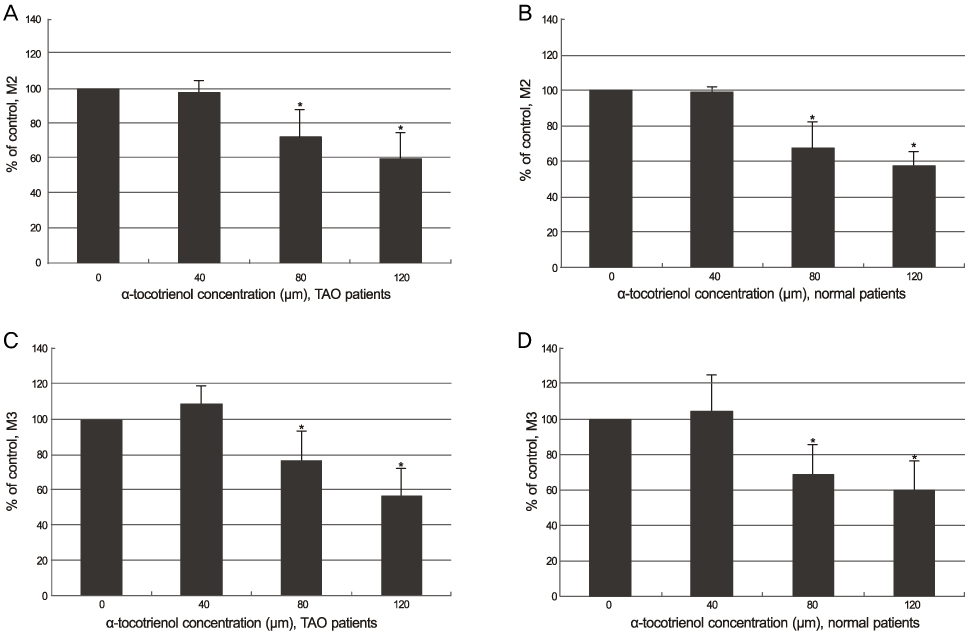
The alpha-tocotrienol and alpha-tocopherol groups showed no cytotoxicity up to 150 microm in orbital fibroblasts from TAO and normal patients. The proliferation of orbital fibroblasts from TAO and normal patients was significantly inhibited with alpha-tocotrienol at 80 microm and 120 microm. The collagen production of orbital fibroblasts from TAO patients was significantly inhibited with alpha-tocotrienol at 120 microm.
CONCLUSIONS
The results from the present study indicate that non-toxic concentrations of alpha-tocotrienol have significant antiproliferative and antifibrotic effects on orbital fibroblasts from TAO patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Garrity JA, Bahn RS. Pathogenesis of graves ophthalmopathy: implications for prediction, prevention, and treatment. Am J Ophthalmol. 2006. 142:147–153.2. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994. 92:477–588.3. Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010. 362:726–738.4. Bahn RS, Heufelder AE. Pathogenesis of Graves' ophthalmopathy. N Engl J Med. 1993. 329:1468–1475.5. Brix TH, Kyvik KO, Hegedüs L. What is the evidence of genetic factors in the etiology of Graves' disease? A brief review. Thyroid. 1998. 8:727–734.6. Weetman AP, McGregor AM. Autoimmune thyroid disease: further developments in our understanding. Endocr Rev. 1994. 15:788–830.7. Hägg E, Asplund K. Is endocrine ophthalmopathy related to smoking? Br Med J (Clin Res Ed). 1987. 295:634–635.8. Tomer Y, Davies TF. Infection, thyroid disease, and autoimmunity. Endocr Rev. 1993. 14:107–120.9. Bahn RS, Dutton CM, Natt N, et al. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998. 83:998–1002.10. Smith TJ, Bahn RS, Gorman CA, Cheavens M. Stimulation of glycosaminoglycan accumulation by interferon gamma in cultured human retroocular fibroblasts. J Clin Endocrinol Metab. 1991. 72:1169–1171.11. Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995. 268:C382–C388.12. Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-1 receptor. J Clin Endocrinol Metab. 2004. 89:5076–5080.13. van Steensel L, Dik WA. The orbital fibroblast: a key player and target for therapy in graves' ophthalmopathy. Orbit. 2010. 29:202–206.14. Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997. 151:317–322.15. Fries KM, Blieden T, Looney RJ, et al. Evidence of fibroblast heterogeneity and the role of fibroblast subpopulations in fibrosis. Clin Immunol Immunopathol. 1994. 72:283–292.16. Sempowski GD, Rozenblit J, Smith TJ, Phipps RP. Human orbital fibroblasts are activated through CD40 to induce proinflammatory cytokine production. Am J Physiol. 1998. 274:C707–C714.17. Powers CJ, MeLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000. 7:165–197.18. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid- associated ophthalmopathy. J Clin Endocrinol Metab. 2002. 87:385–392.19. Hwang CJ, Afifiyan N, Sand D, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009. 50:2262–2268.20. Lehmann GM, Feldon SE, Smith TJ, Phipps RP. Immune mechanisms in thyroid eye disease. Thyroid. 2008. 18:959–965.21. Lisi S, Botta R, Lemmi M, et al. Quercetin decreases proliferation of orbital fibroblasts and their release of hyaluronic acid. J Endocrinol Invest. 2011. 34:521–527.22. Kim H, Choi YH, Park SJ, et al. Antifibrotic effect of Pirfenidone on orbital fibroblasts of patients with thyroid-associated ophthalmopathy by decreasing TIMP-1 and collagen levels. Invest Ophthalmol Vis Sci. 2010. 51:3061–3066.23. Tappeiner C, Meyenberg A, Goldblum D, et al. Antifibrotic effects of tocotrienols on human Tenon's fibroblasts. Graefes Arch Clin Exp Ophthalmol. 2010. 248:65–71.24. Meyenberg A, Goldblum D, Zingg JM, et al. Tocotrienol inhibits proliferation of human Tenon's fibroblasts in vitro: a comparative study with vitamin E forms and mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2005. 243:1263–1271.25. Mishima K, Tanaka T, Pu F, et al. Vitamin E isoforms alpha-tocotrienol and gamma-tocopherol prevent cerebral infarction in mice. Neurosci Lett. 2003. 337:56–60.26. Unchern S, Laoharuangpanya N, Phumala N, et al. The effects of vitamin E on platelet activity in beta-thalassaemia patients. Br J Haematol. 2003. 123:738–744.27. Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003. 17:816–822.28. Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hypercholesterolemic humans. Atherosclerosis. 2002. 161:199–207.29. Tomeo AC, Geller M, Watkins TR, et al. Antioxidant effects of tocotrienols in patients with hyperlipidemia and carotid stenosis. Lipids. 1995. 30:1179–1183.30. Malafa MP, Fokum FD, Mowlavi A, et al. Vitamin E inhibits melanoma growth in mice. Surgery. 2002. 131:85–91.31. Nesaretnam K, Guthrie N, Chambers AF, Carroll KK. Effect of tocotrienols on the growth of a human breast cancer cell line in culture. Lipids. 1995. 30:1139–1143.32. Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991. 266:6188–6194.33. Kline K, Yu W, Sanders BG. Vitamin E: mechanisms of action as tumor cell growth inhibitors. J Nutr. 2001. 131:161–163.34. Tasinato A, Boscoboinik D, Bartoli GM, et al. d-alpha-tocopherol inhibiton of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci U S A. 1995. 92:12190–12194.35. Haas AL, Boscoboinik D, Mojon DS, et al. Vitamin E inhibits proliferation of human Tenon's capsule fibroblasts in vitro. Ophthalmic Res. 1996. 28:171–175.36. Haas AL, Boscoboinik DO, Bohnke M. Inhibition of Tenon fibroblast proliferation. Comparison between tocopherol and mitomycin-C. Invest Ophthalmol Vis Sci. 1996. 37:Suppl. 877S.37. Larrosa JM, Veloso AA Jr, Leong FL, Refojo MF. Antiproliferative effect of intravitreal alpha-tocopherol and alpha-tocopheryl-acid-succinate in a rabbit model of PVR. Curr Eye Res. 1997. 16:1030–1035.38. Larrosa JM, Polo V, Ramirez T, et al. Alpha-tocopherol derivatives and wound healing in an experimental model of filtering surgery. Ophthalmic Surg Lasers. 2000. 31:131–135.39. Mojon D, Boscoboinik D, Haas A, et al. Vitamin E inhibits retinal pigment epithelium cell proliferation in vitro. Ophthalmic Res. 1994. 26:304–309.40. Pinilla I, Larrosa JM, Polo V, Honrubia FM. Alpha-tocopherol derivatives in an experimental model of filtering surgery. Ophthalmic Res. 1999. 31:440–445.41. Pinilla I, Piazuelo E, Jiménez P, et al. Inhibitory effect of alpha tocopherol succinate on fibroblast wound healing. Arch Soc Esp Oftalmol. 2000. 75:383–388.42. Sakamoto T, Hinton DR, Kimura H, et al. Vitamin E succinate inhibits proliferation and migration of retinal pigment epithelial cells in vitro: therapeutic implication for proliferative vitreoretinopathy. Graefes Arch Clin Exp Ophthalmol. 1996. 234:186–192.43. Sakamoto T, Oshima Y, Ishibashi T, Inomata H. Inhibitory effect of Vitamin E succinate on the proliferation of cultured bovine choroidal endothelial cells. Nihon Ganka Gakkai Zasshi. 1996. 100:777–782.44. Sylvester PW, McIntyre BS, Gapor A, Briski DP. Vitamin E inhibition of normal mammary epithelial cell growth is associated with a reduction in protein kinase C(alpha) activation. Cell Prolif. 2001. 34:347–357.45. Kim JE, Park JW, Cho JK, Yoon KC. Therapeutic effects of periocular injection of triamcinolon acetonide in patients with thyroid-associated ophthalmopathy. J Korean Ophthalmol Soc. 2011. 52:788–793.46. Kim JH, Lee TS. A study of factors related to the course of Graves' ophthalmopathy. J Korean Ophthalmol Soc. 2011. 52:255–260.47. Jung BY, Kim YD. The results of periocular injections of triamcinolone for thyroid orbitopathy. J Korean Ophthalmol Soc. 2007. 48:1163–1169.48. Lee JH, Lee SY, Yoon JS. Risk factors associated with the severity of thyroid-associated orbitopathy in Korean patients. Korean J Ophthalmol. 2010. 24:267–273.49. Chu YK, Kim SJ, Lee SY. Surgical treatment modalities of thyroid ophthalmopathy. Korean J Ophthalmol. 2001. 15:128–132.50. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008. 181:4397–4405.51. Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003. 170:6348–6354.52. Gianoukakis AG, Khadavi N, Smith TJ. Cytokines, Graves' disease, and thyroid-associated ophthalmopathy. Thyroid. 2008. 18:953–958.53. Smith TJ. Unique properties of orbital connective tissue underlie its involvement in Graves' disease. Minerva Endocrinol. 2003. 28:213–222.54. Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008. 214:199–210.55. Pihlajaniemi T, Myllylä R, Kivirikko KI. Prolyl 4-hydroxylase and its role in collagen synthesis. J Hepatol. 1991. 13:Suppl 3. S2–S7.56. Yao HW, Zhu JP, Zhao MH, Lu Y. Losartan attenuates bleomycin-induced pulmonary fibrosis in rats. Respiration. 2006. 73:236–242.57. van Steensel L, Paridaens D, Schrijver B, et al. Imatinib mesylate and AMN107 inhibit PDGF-signaling in orbital fibroblasts: a potential treatment for graves' ophthalmopathy. Invest Ophthalmol Vis Sci. 2009. 50:3091–3098.58. Distler JH, Jüngel A, Huber LC, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007. 56:311–322.59. Akhmetshina A, Dees C, Pileckyte M, et al. Dual inhibition of c-abl and PDGF receptor signaling by dasatinib and nilotinib for the treatment of dermal fibrosis. FASEB J. 2008. 22:2214–2222.60. Bartalena L, Tanda ML, Piantanida E, Lai A. Oxidative stress and Graves' ophthalmopathy: in vitro studies and therapeutic implications. Biofactors. 2003. 19:155–163.61. Burch HB, Lahiri S, Bahn RS, Barnes S. Superoxide radical production stimulates retroocular fibroblast proliferation in Graves' ophthalmopathy. Exp Eye Res. 1997. 65:311–316.62. Hondur A, Konuk O, Dincel AS, et al. Oxidative stress and antioxidant activity in orbital fibroadipose tissue in Graves' ophthalmopathy. Curr Eye Res. 2008. 33:421–427.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Gangliosides Mixture on Differentiation of Orbital Fibroblasts into Adipocytes
- The Effect of Previous Orbital Decompression on Outcome of Strabismus Surgery in Patients with Thyroid Ophthalmopathy
- Three Wall Orbital Decompression for Compressive Optic Neuropathy in Thyroid Ophthalmopathy
- Surgical treatment modalities of thyroid ophthalmopathy
- Benzylideneacetophenone derivatives attenuate IFN-gamma-induced IP-10/CXCL10 production in orbital fibroblasts of patients with thyroid-associated ophthalmopathy through STAT-1 inhibition