J Korean Ophthalmol Soc.
2010 Dec;51(12):1652-1658.
Effective Keratocyte Culture Using Amniotic Membrane Matrix and Differentiation of Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Ophthalmology, Chung-Ang University College of Medicine, Seoul, Korea. jck50ey@kornet.net
Abstract
- PURPOSE
To investigate the characteristics of cultured rabbit corneal keratocytes in vitro and evaluate the possibility of differentiation of mesenchymal stem cells to keratocytes using the keratocyte conditioned medium (KCM).
METHODS
Isolated keratocytes were seeded on the stromal side of amniotic membranes (AM) or plastic dishes, and morphologic changes were evaluated. Rabbit mesenchymal stem cells were cultured on AM with alpha-MEM (minimum essential medium alpha) and KCM. The gene expression patterns of specific keratocyte markers (keratocan, lumican, and aldehyde dehydrogenase family, member A1 (ALDH1A1)) of cultured cells were evaluated by RT-PCR.
RESULTS
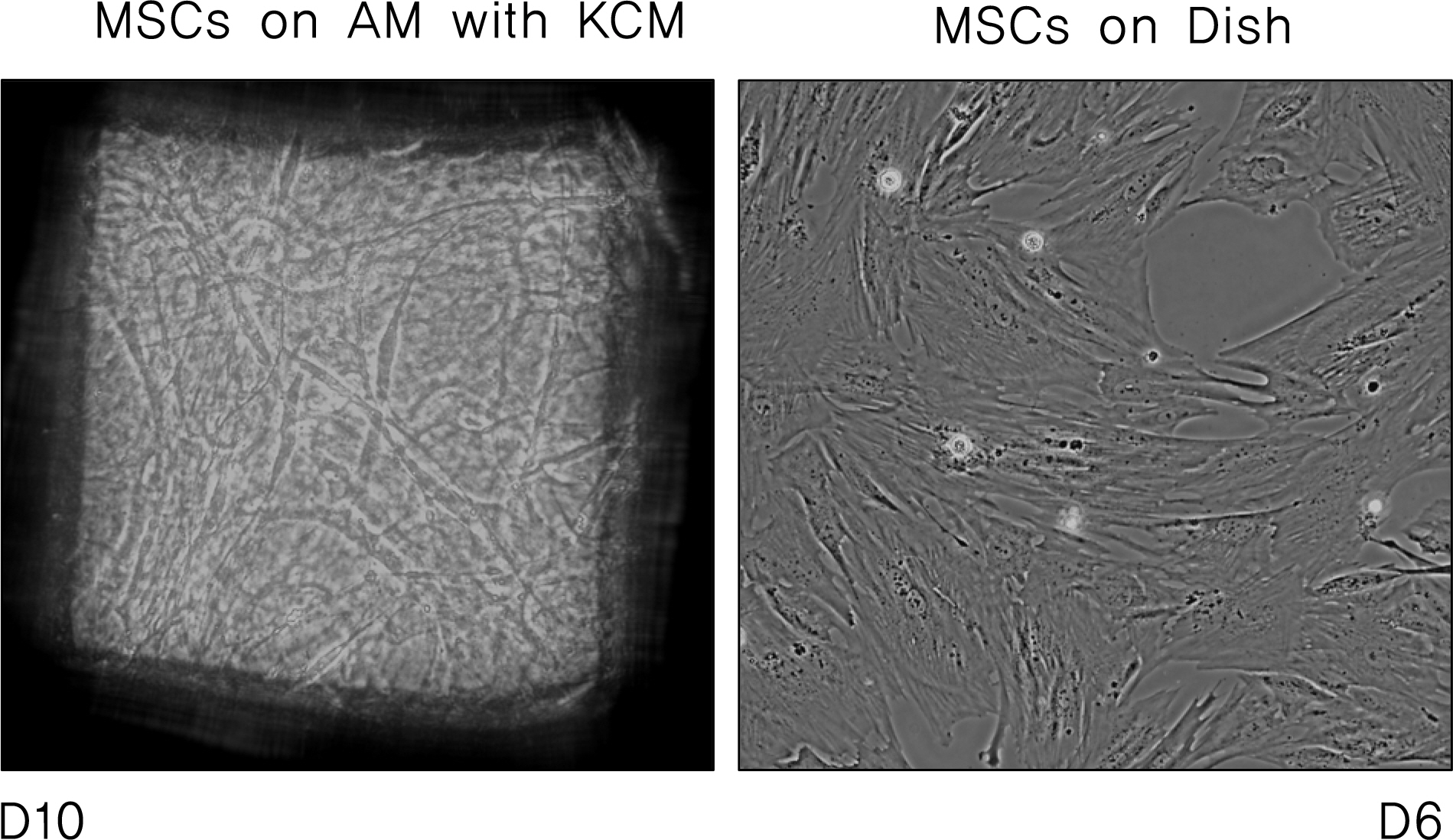
Keratocytes on AM showed dendritic morphology with slow proliferation in contrast, cells on dishes were stellate in shape with fast proliferation. Cultured keratocytes on AM maintained the expression of keratocan, lumican and ALDH1A1 while keratocytes on plastic dishes steadily lost their keratocyte marker gene expression. Additionally, mesenchymal stem cells cultured with KCM on AM induced expression of keratocan and ALDH1A1.
CONCLUSIONS
Keratocytes cultured on AM stromal matrix maintained their characteristic morphology and marker gene expression. Morphology changes and marker gene expressions of mesenchymal stem cells suggest an ability to differentiate into keratocytes when grown on AM with KCM.
Keyword
MeSH Terms
-
Aldehyde Dehydrogenase
Amnion
Cells, Cultured
Chondroitin Sulfate Proteoglycans
Corneal Keratocytes
Culture Media, Conditioned
Gene Expression
Humans
Keratan Sulfate
Mesenchymal Stromal Cells
Organic Chemicals
Plastics
Seeds
Aldehyde Dehydrogenase
Chondroitin Sulfate Proteoglycans
Culture Media, Conditioned
Keratan Sulfate
Organic Chemicals
Plastics
Figure
Reference
-
References
11. Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001; 128:5181–8.
Article12. Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998; 279:1528–30.
Article13. Okamoto R, Yajima T, Yamazaki M, et al. Damaged epithelia regenerated by bone marrow-derived cells in the human gastro-intestinal tract. Nat Med. 2002; 8:1011–7.
Article14. Wei ZG, Wu RL, Lavker RM, Sun T. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Invest Ophthalmol Vis Sci. 1993; 34:1814–28.15. Chung E, Bukusoglu G, Zieske JD. Localization of corneal epithelial stem cells in the developing rat. Invest Ophthalmol Vis Sci. 1992; 33:2199–206.16. Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999; 179:325–35.17. Koizumi NJ, Inatomi TJ, Sotozono CJ, et al. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000; 20:173–7.
Article18. Wong HC, Boulton M, Marshall J, Clark P. Growth of retinal capillary endothelia using pericyte conditioned medium. Invest Ophthalmol Vis Sci. 1987; 28:1767–75.19. Razin E, Cordon-Cardo C, Good RA. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981; 78:2559–61.
Article20. Gho YS, Kim PN, Li HC, et al. Stimulation of tumor growth by human soluble intercellular adhesion molecule-1. Cancer Res. 2001; 61:4253–7.21. Hall PA, Watt FM. The generation and maintenance of cellular diversity. Development. 1989; 106:619–33.22. Lajtha LG. Stem cell concepts. Differentiation. 1979; 14:23–34.
Article23. Kicic A, Shen W, Rakoczy PE. The potential of marrow stromal cells in stem cell therapy. Eye. 2001; 15:695–707.
Article24. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19:180–92.
Article25. Ishizaki M, Wakamatsu K, Matsunami T, et al. Dynamics of the expression of cytoskeleton components and adherens molecules by fibroblastic cells in alkali-burned and lacerated corneas. Exp Eye Res. 1994; 59:537–49.
Article26. Nakamura K, Kurosaka D, Yoshino M, et al. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts. Invest Ophthalmol Vis Sci. 2002; 43:2603–8.27. Djouad F, Delorme B, Maurice M, et al. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007; 9:R33.
Article28. Metallo CM, Mohr JC, Detzel CJ, et al. Engineering the stem cell microenvironment. Biotechnol Prog. 2007; 23:18–23.
Article29. Shin MS, Hong HS, Son YS, Kim JC. Effects of Damaged Human Corneal Epithelial Cells on Differentiation of Human Mesenchymal Stem Cell. J Korean Ophthalmol Soc. 2007; 48:423–30.30. Choong PF, Mok PL, Cheong SK, Then KY. Mesenchymal stromal cell-like characteristics of corneal keratocytes. Cytotherapy. 2007; 9:252–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Strategy of Ocular Surface Disease: Ocular Surface Reconstruction Using Amniotic Membrane and Limbal Stem Cell Transplantation
- Comparison with human amniotic membrane- and adipose tissue-derived mesenchymal stem cells
- Effect of UV-B and Amniotic Membrane on Inflammation, Lipid Peroxidation and Keratocyte Apoptosis Induced by PRK
- A Mini Overview of Isolation, Characterization and Application of Amniotic Fluid Stem Cells
- Isolation and characterization of equine amniotic membrane-derived mesenchymal stem cells