J Korean Soc Clin Pharmacol Ther.
2013 Jun;21(1):34-40.
Pharmacodynamic Comparison of Two Formulations of Voglibose 0.3-mg Tablet
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, Seoul, Korea. ksbae@amc.seoul.kr
- 2University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Clinical Pharmacology, Inha University Hospital, Inha University School of Medicine, Incheon, Korea.
Abstract
- BACKGROUND
Voglibose, an inhibitor of alpha-glucosidase of the small intestine brush border, is used to treat type 2 diabetic patients. Bioequivalence test based on pharmacokinetic parameters is difficult because voglibose does not cross the enterocytes after ingestion. This study was conducted to establish bioequivalence of two formulations of 0.3-mg voglibose with pharmacodynamic endpoints.
METHODS
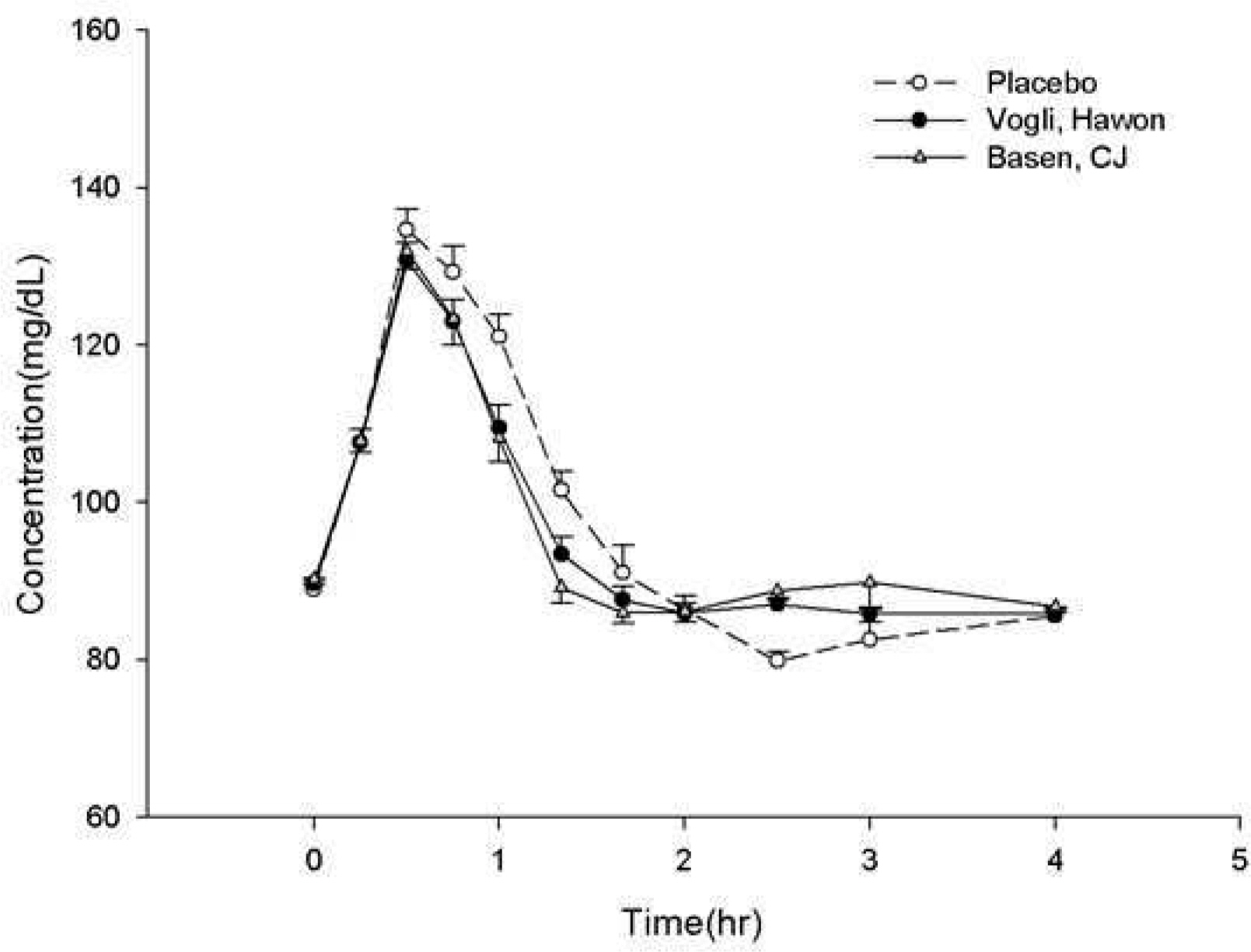
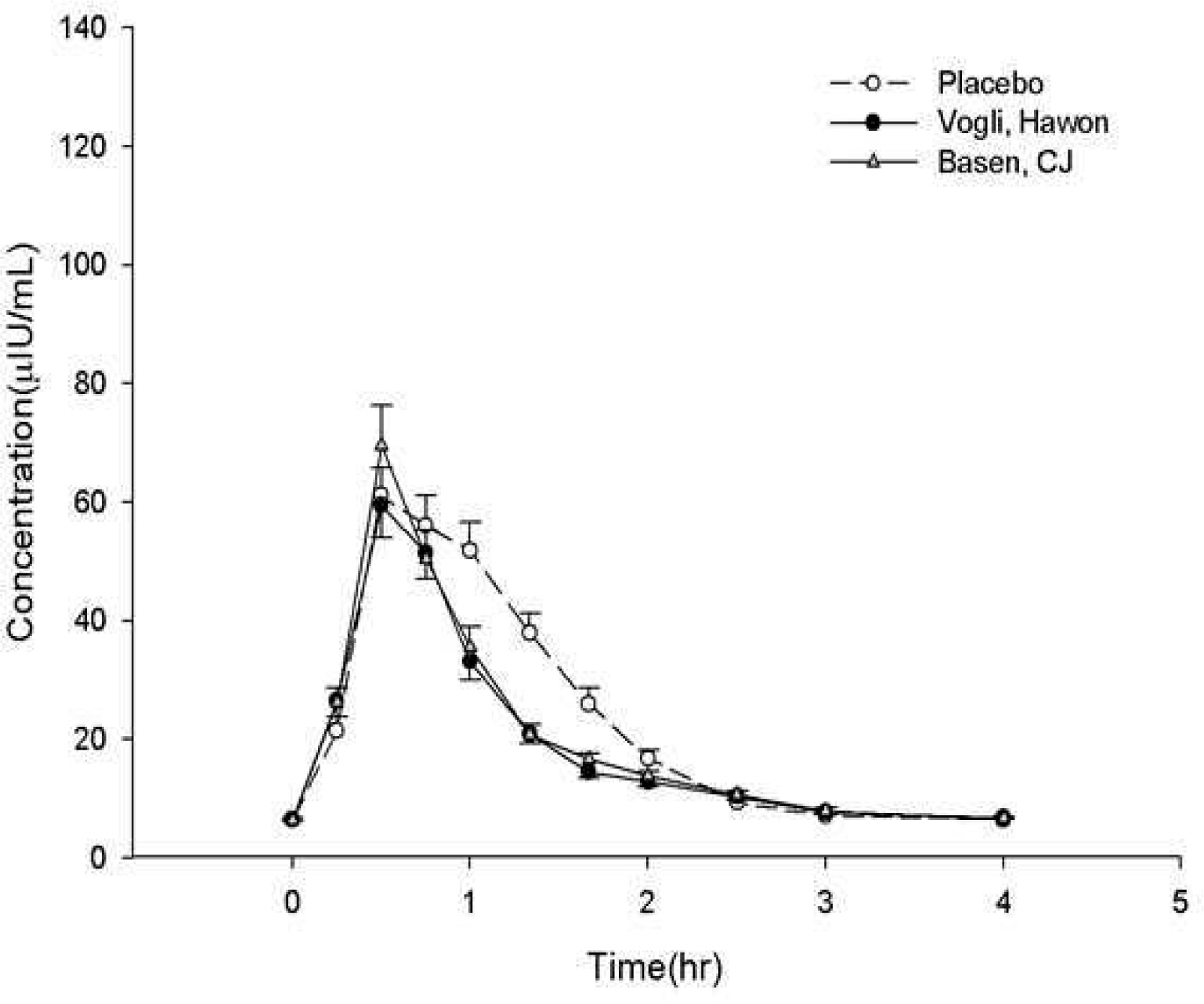
This study was an open, single-dose, randomized, 6-sequence, 3-period crossover design in healthy volunteers. In each period, subjects received placebo or three tablets of either test formulation or reference formulation with sucrose, with a 7-day washout period each dosing period. Serial blood samples were collected after each administration. The maximum concentrations of serum glucose and serum insulin (C(max)(G) and C(max)(I)) and the area under the serum concentration - time curve from dosing to 2 or 4 hours after dosing for serum glucose and insulin (AUC(0-2h)(G), AUC(0-4h)(G), AUC(0-2h)(I) and AUC(0-4h)(I), respectively) were determined by noncompartmental analysis. Formulation-related differences were tested in accordance with the Korean regulatory bioequivalence criteria.
RESULTS
A total of 54 subjects completed study in accordance with protocol. The geometric mean ratios (GMRs) of the test formulation to the reference formulation for Cmax(G), AUC(0-2h)(G), AUC(0-4h)(G), C(max)(I), AUC(0-2h)(I) and AUC(0-4h)(I) were 0.945, 1.014, 0.995, 0.937, 0.985 and 0.983, respectively and the 90% confidence intervals (CIs) of corresponding values were 0.985-1.026, 0.991-1.038, 0.977-1.014, 0.830-1.057, 0.901-1.078 and 0.911-1.014, respectively.
CONCLUSION
This single-dose study found that two formulations of 0.3-mg voglibose did not meet the regulatory criteria for bioequivalence in these healthy volunteers.
Keyword
MeSH Terms
Figure
Reference
-
1. Vichayanrat A, Ploybutr S, Tunlakit M, Watanakejorn P. Efficacy and safety of voglibose in comparison with acarbose in type 2 diabetic patients. Diabetes Res Clin Pr. 2002; 55(2):99–103.
Article2. Temelkova-Kurktschiev TS, Koehler C, Henkel E, Leonhardt W, Fuecker K, Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA(1c) level. Diabetes Care. 2000; 23(12):1830–1834.3. Global Guideline for Type 2 Diabetes. International Diabetes Federation;2012.4. Oak W. Bioavailability and Bioequivalence Studies for Orally Administered DrugProducts - General Considerations. US Food and Drug Administration;2003.5. Draft Guidance on Acarbose. US Food and Drug Administration;2009.6. Cramp DG. New automated method for measuring glucose by glucose oxidase. J Clin Path. 1967; 20:910–912.
Article7. Zhang M, Yang J, Tao L, Li L, Ma P, Fawcett JP. Acarbose bioequivalence: exploration of new pharmacodynamic parameters. AAPS J. 2012; 14(2):345–351.
Article8. Kageyama S, Nakamichi N, Sekino H, Nakano S. Comparison of the effects of acarbose and voglibose in healthy subjects. Clin Ther. 1997; 19(4):720–729.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Placebo-Controlled, Single and Multiple Dose Study to Investigate the Appropriate Parameters for Evaluation of Pharmacodynamic Equivalence of Voglibose in Healthy Korean Volunteers
- Role of Intestinal Microbiota in Metabolism of Voglibose In Vitro and In Vivo
- Pharmacokinetic comparison of two valproic acid formulations: a plain and a controlled release enteric-coated tablets
- Bioequivalence and Dose Proportionality of Olmesartan Medoxomil Formulations
- Comparative pharmacokinetic and tolerability evaluation of two simvastatin 20 mg formulations in healthy Korean male volunteers