J Korean Soc Spine Surg.
2001 Dec;8(4):455-467.
Comparison of Osteosyntheses in Various Types of Porous Calcium Phosphate Compounds: An Experimental Study by Posterolateral Fusion of Rabbits' Lumbar Vertebrae
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University Medical College, Seoul, Korea. choonki@plaza.snu.ac.kr
- 2School of Materials Science & Engineering, Seoul National University, Seoul, Korea.
- 3Bioalpha.com, Seoul, Korea.
Abstract
-
PURPOSE: The purpose of the present study was to compare the osteoconduction in porous bodies made of various compositions of calcium phosphate compounds and other porous artificial bones.
MATERIALS AND METHODS
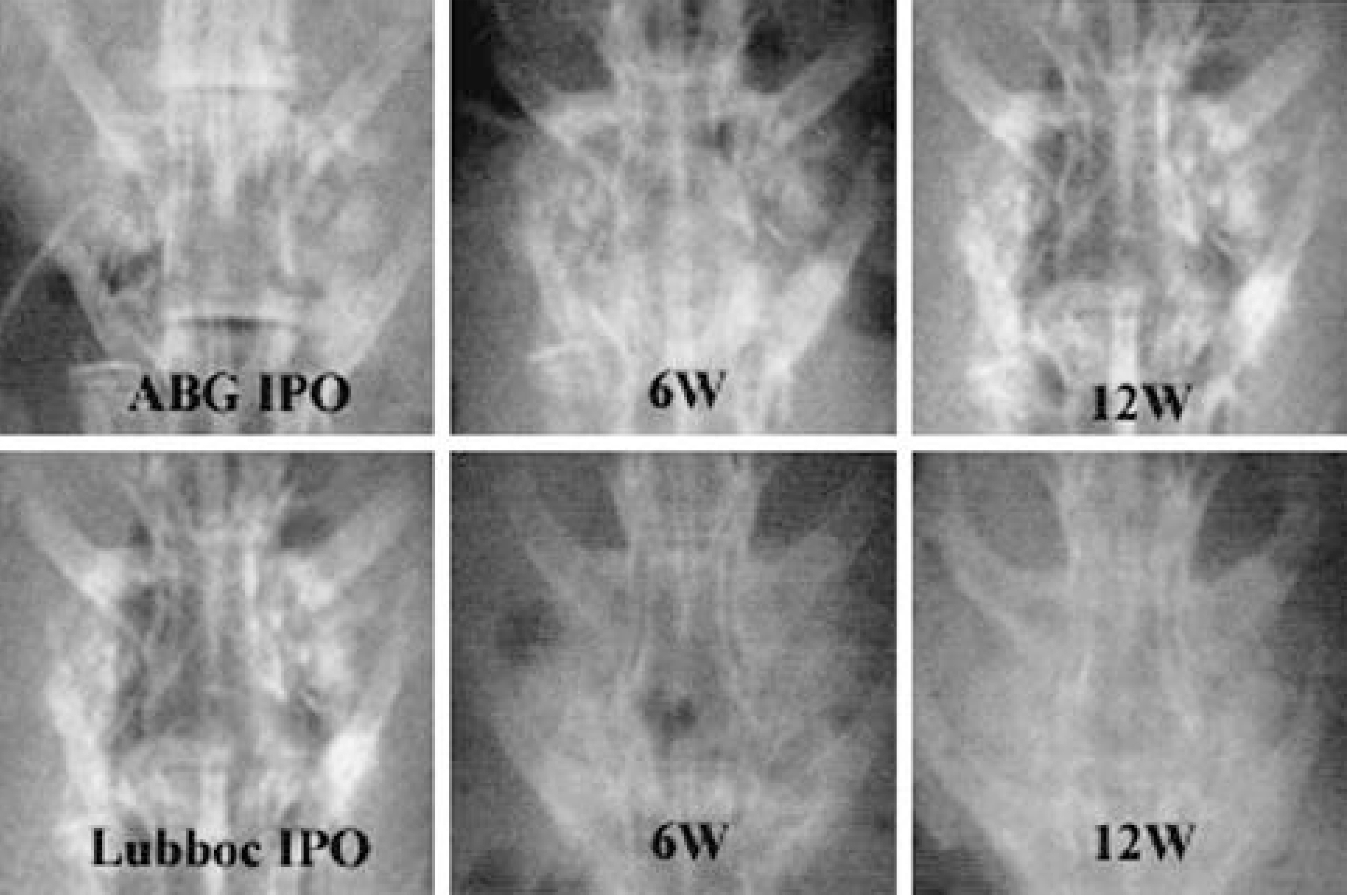
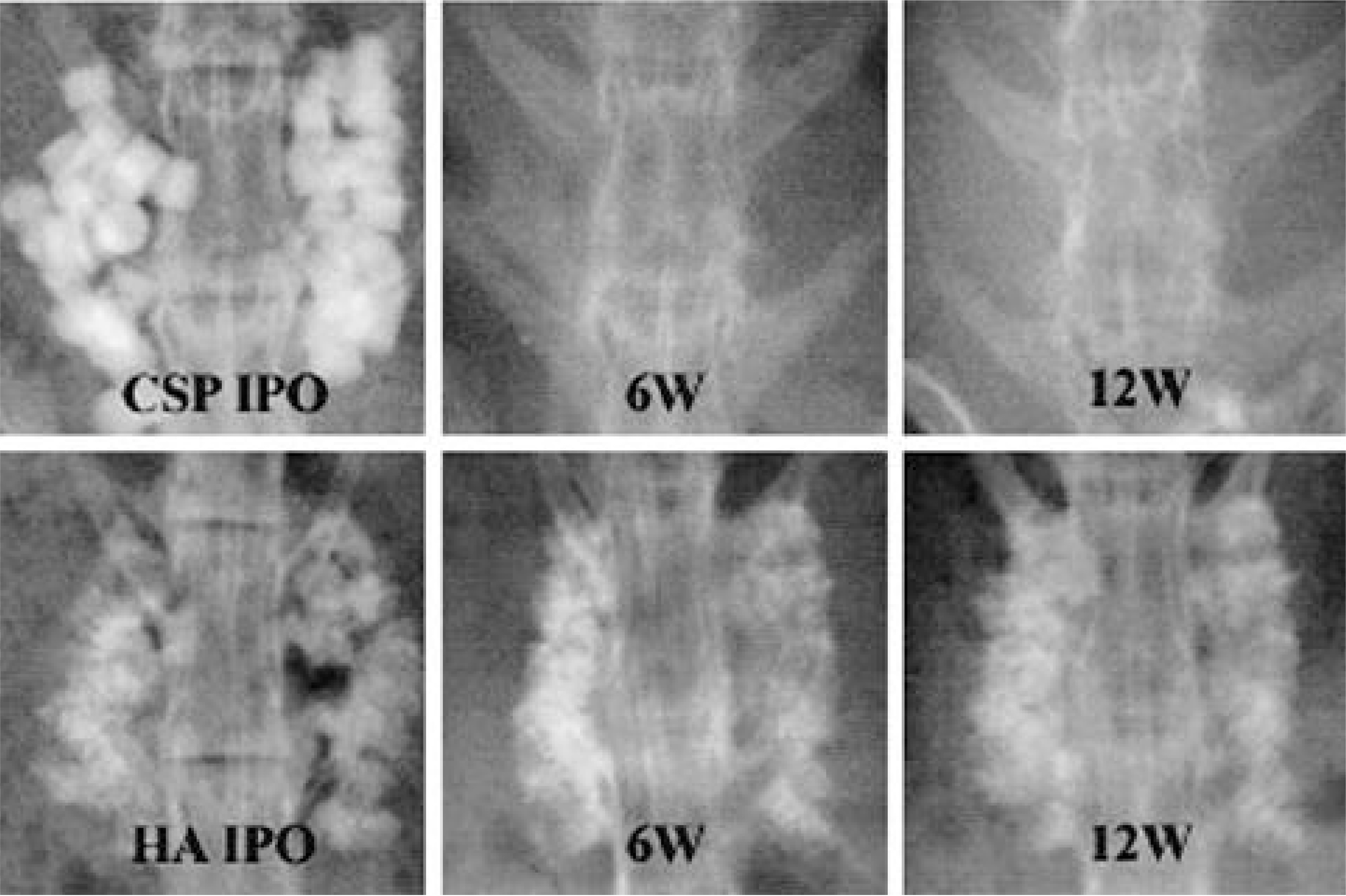
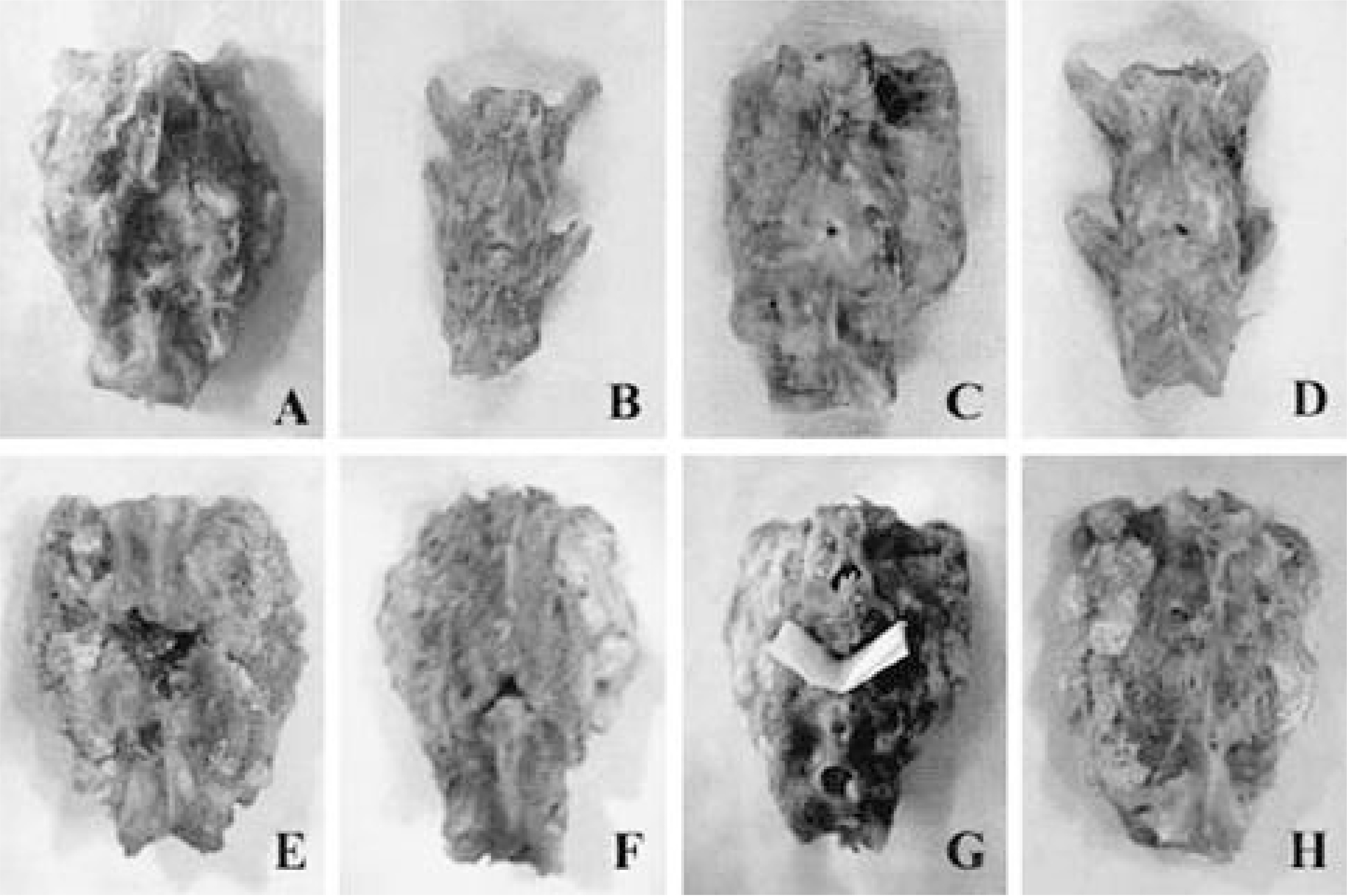
Single-level posterolateral spinal fusions were performed on ninety rabbits. The animals were divided into nine groups by graft materials: autograft (positive control), implantation of HA, TCP, CPP, HA/TCP composite, TCP/CPP composite, Lubboc(R) and Calcium sulfate pellet (CSP), no-graft after decortication (negative control). Serial radiography, serum calcium and phosphorus levels were checked. All animals were sacrificed 12 weeks after surgery and the fusion masses were compared by manual palpation, uniaxial tensile strength measurement and histological evaluation.
RESULTS
Autografted and CPP implanted groups showed significantly higher fusion ratio than TCP, TCP/CPP composite, and no-graft groups. Meanwhile, HA and HA/TCP groups showed no significant difference with other groups in fusion ratio. From the radiological examination, TCP and CPP groups seemed to show more rapid absorption of implant than HA group. The mean values of tensile strength of autografted and CPP group were significantly larger than those of TCP, TCP/CPP composite, and no-graft groups. The result of direct inspection and microscopic examination showed the TCP-contained implants lost their porous structure, whereas the other implants did not. On the light microscopy, both HA and CPP groups showed more abundant new bone growth into the pores than TCP-contained groups, but the pore size of CPP became larger than that of the HA, which manifested more rapid absorption of CPP in the living body.
CONCLUSION
The porous CPP implant is considered to be more desirable bone graft substitute because it has satisfactory osteoconductive ablility and better biodegradation than porous HA. And the maintenance of porous structure is considered to be indispensable for osteoconduction.
MeSH Terms
Figure
Reference
-
1). Boden SD, Schimandle H, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 20:412–20. 1995.2). Bucholz RW, Carlton A, Holmes RE. Hydroxyapatite and tricalcium phosphate bone graft substitute. Orthop Clin North Am. 18:323–334. 1987.3). Chung SS, Hong KS, Youn HJ, Chang BS, Yeom JS, Lee CK, Park YK, Ryu HS, Park KW. Effects of Pore Size on Osteoconduction at the Porous Hydroxyapatite. J Korean Orthop Assoc. 33:158–167. 1998.
Article4). De Aza PN, Guitian F, De Aza S. Bioeutectic: a new ceramic material for human bone replacement. Biomaterials. 18(19):1285–1291. 1997.
Article5). Freidlaender GE. Current concepts review: Bone grafts. J Bone Joint Surg. 69-A:786–790. 1987.6). Hench LL. Bioceramics. From concept to clinic. J Am Ceram Soc. 74(7):1487–1510. 1991.
Article7). Holmes RE, Bucholz RW, Monney V. Porous hydroxyapatite as a bonegraft substitute in metaphyseal defects. J Bone Joint Surg. 68-A:904–911. 1986.8). Ishihara K, Arai H, Nakabayashi N, Morita S, Fu-ruya K. Adhesive bone cement containing hydroxyapatite particle as bone compatible filler. J Biomed Mater Res. 26:937–945. 1992.
Article9). Ishikawa K, Ducheyne P, Radin S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J mater Sci Med. 4:165–168. 1993.
Article10). Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mat Res. 17:769–784. 1983.
Article11). Lee CK, Cho KH, Suh H, Ahn SJ. A radiological and histological study of carbonate apatite collagen composite and hydroxyapatite implanted in bone defects of the rabbit tibiae. J Korean Orthop Assoc. 30:1109–1118. 1995.
Article12). Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell substra -tum controls BMP-induced osteogenesis. J Biochem. 121:317–324. 1997.13). Yamamura K, Iwata H, Yotsuyanagi T. Synthesis of antibiotic-loaded hydroxyapatite beads and in vitro drug release testing. J Biomed Mater Res. 26:1053–1064. 1992.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Bone Union Rates Using a Local Autobone and Bone Graft Substitute Mixed Graft in Lumbar Posterolateral Fusion
- Union Rates of Autologous Bone Marrow, Local Autobone and Biphasic Calcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- Biphasic Calcium Phosphate and Local Autobone Mixed Graft in Lumbar Posterolateral Fusion
- Union Rates of Local Autobone and beta-Tricalcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- The First Clinical Trial of Beta-Calcium Pyrophosphate as a Novel Bone Graft Extender in Instrumented Posterolateral Lumbar Fusion