J Korean Soc Spine Surg.
2013 Sep;20(3):71-76. 10.4184/jkss.2013.20.3.71.
Union Rates of Local Autobone and beta-Tricalcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- Affiliations
-
- 1Department of Orthopedic Surgery, Busan Medical Center, Busan, Korea. panggul@naver.com
- KMID: 1487971
- DOI: http://doi.org/10.4184/jkss.2013.20.3.71
Abstract
- STUDY DESIGN: A retroprospective study.
OBJECTIVES
We used a local autobone and beta-tricalcium phosphate mixed graft with posterolateral fusion in spinal stenosis and spondylolisthesis and evaluated union rates to verify the efficacy. SUMMARY OF LITERATURE REVIEW: Several reports have shown high union rates of posterolateral fusion using beta-tricalcium phosphate. However, in Korea, only one study reported a low union rate.
MATERIALS AND METHODS
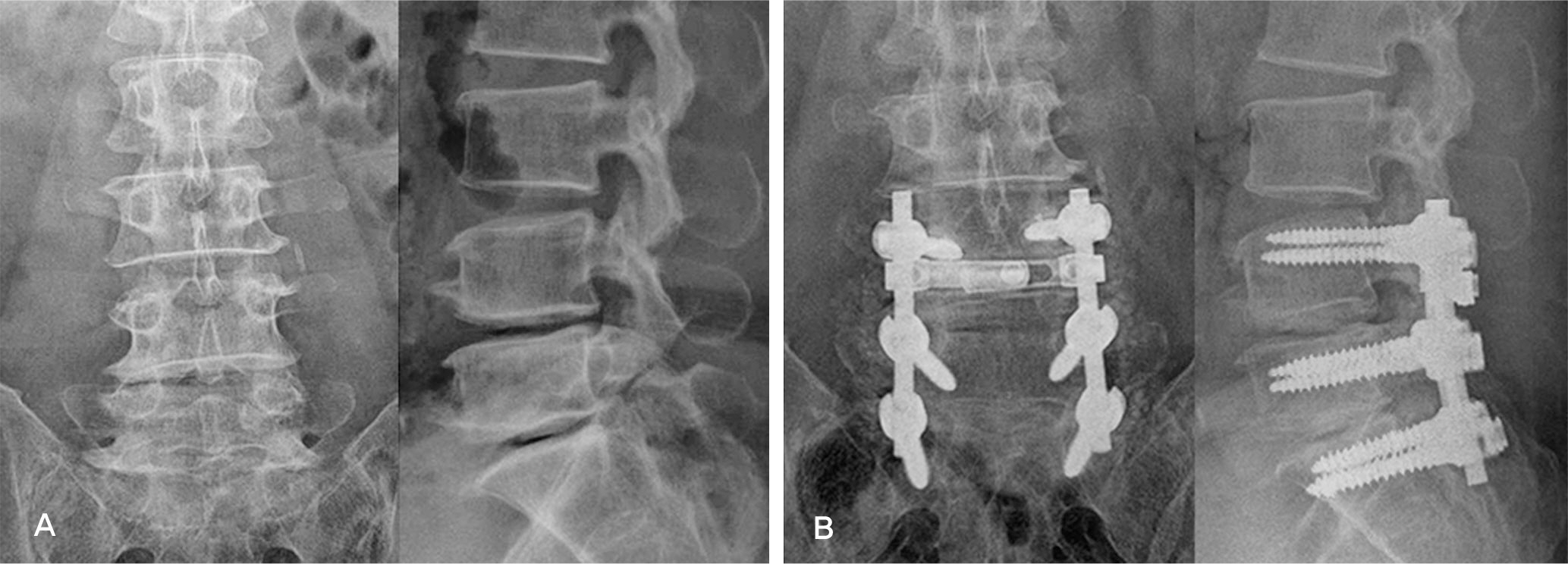
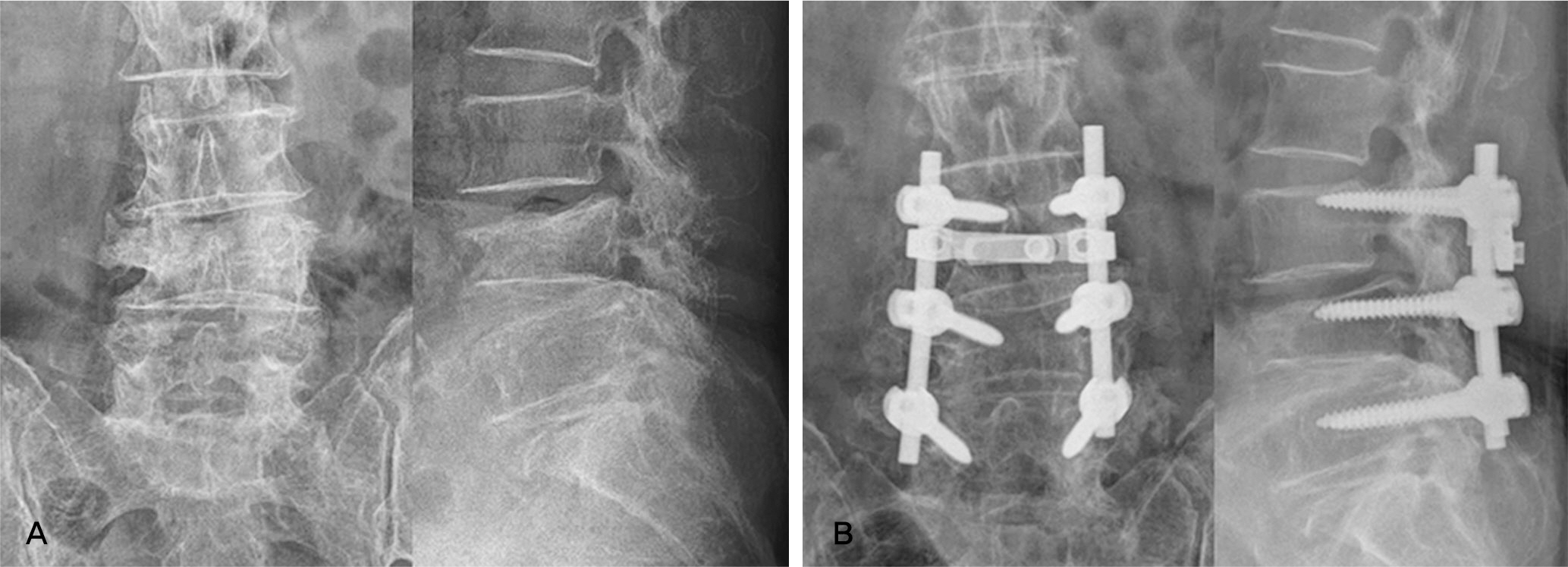
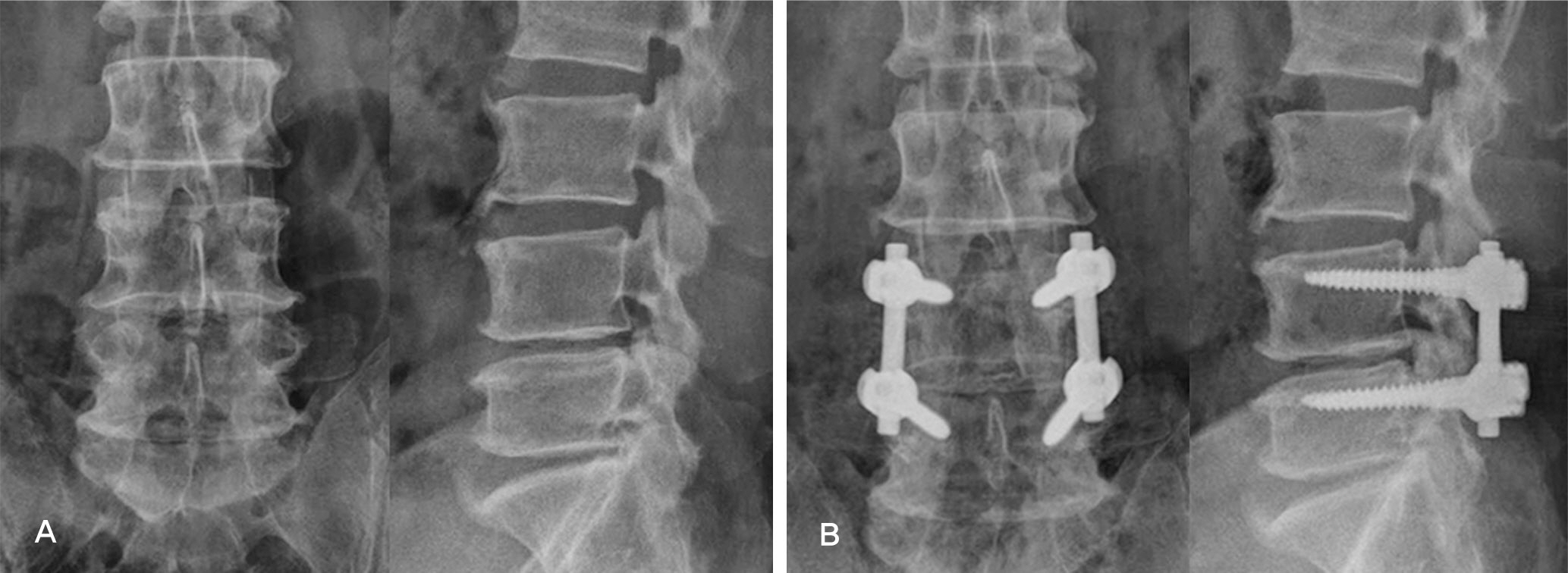
Forty-two patients who underwent lumbar posterolateral fusion with a local autobone and beta-tricalcium phosphate mixed graft from September 2010 to July 2011 were followed up. There were 32 cases with spinal stenosis and 10 cases with spondylolisthesis. Bone fusion was determined along with the fusion rates based on Lenke's criteria. Clinical outcomes were determined using Kim's method.
RESULTS
In spinal stenosis, bone union was presented in 19 cases(59.4%) out of 32 cases and in spondylolisthesis, bone union was presented in 7 (70.0%) out of 10. In spinal stenosis, 12 cases showed excellent outcome, 16 good, 3 fair and 1 poor, 27 cases(87.5%) were superior to the good. In spondylolisthesis, 2 cases showed excellent outcome, 5 good, 3 fair and 0 poor, 8 cases(70.0%) were superior to the good.
CONCLUSIONS
Posterolateral fusion using a local autobone and beta-tricalcium phosphate mixed graft showed lower bone fusion rates. We need further studies to enhance the fusion rate when using local autobone and beta-tricalcium phosphate mixed grafts.
MeSH Terms
Figure
Reference
-
1. Watkins MB. Posterolateral fusion of the lumbar and lumbosacral spine. J Bone Joint Surg. 1953; 35(1014):8.
Article2. Jeon CH, Kim YC, Chang HG, Kim YW, Jung NS, Shin DS. The comparison of changes in the dimensions of the intervertebral disc and neural foramen between anterior lumbar interbody fusion and posterolateral fusion in the lumbar spine. J Kor Soc Spine surg. 2007; 14:263–9.
Article3. Lee CK, Langrana NA. Lumbosacral spinal fusion. A biomechanical study. Spine (Phila Pa 1976). 1984; 9:574–81.
Article4. Rombold C. Treatment of spondylolisthesis by posterolateral fusion, resection of the pars interarticularis and prompt immobilization of the patients. J Bone Joint Surg. 1966; 48:1282–300.5. Turner JA, Ersek M, Herron L, et al. Patient outcomes after lumbar spinal fusion. JAMA. 1992; 268:907–11.6. Lee SU, Kim KT, Ahn OK, Ok JC. Posterolateral fusion in spondylolisthesis. J of Korean Orthop. 1996; 31:695–701.
Article7. Younger EX, Chapman MW. Morbidity at bone graft donor sites. Orthop Trans. 1986; 10:494.
Article8. Park HJ, Kim WK, Shim YJ, Kang DH. Efficiency of anterior interbody fusion using cage packed with DBM in dis-tractive flexion injury of cervical spine –demineralized bone matrix vs autoiliac cancellous bone-. J Kor Soc Spine surg. 2010; 17:111–9.9. Galois L, Mainard D, Cohen P, Pfeffer F, Traversari R, Delagoutte JP. Filling of bone defects with tricalcium phosphate beta in traumatology. Ann Chir. 2000; 125:972–81.10. Galois L, Mainard D, Delagoutte JP. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int Orthop. 2002; 26:109–15.11. Le Huec JC, Lesprit E, Delavigne C, Clement D, Chauveaux D, Le Rebeller A. Tricalcium phosphate ce-ramics and allografts as bone substitutes for spinal fusion in idiopathic scoliosis comparative clinical results at four years. Acta Orthop Belg. 1997; 63:202–11.12. Cosar M, Ozer AF, Iplikcioglu AC, et al. The results of beta-tricalcium phosphate coated hydroxyapatite (beta-TCP/HA) grafts for interbody fusion after anterior cervical discectomy. J Spinal Disord Tech. 2008; 21:436–41.13. Suk SI. Spinal surgery. 2nded.Newest medical publishing company;2004. p. 126–7.14. Wahba GM, Bhatia N, Bui CN, Lee KH, Lee TQ. Biomechanical evaluation of short-segmental posterior instrumentation with and without crosslinks in a human cadav-eric unstable thoracolumbar burst fracture model. Spine (Phila Pa 1976). 2010; 35:278–85.15. Lenke LG, Bridgwell KH, Baldus C, Blanke K. Contrel-Dubousset instrument tation for adolescent idiopathic scoliosis. J Bone Joint surg Am. 1992; 74:1056–69.16. Kim NH, Lee HM. Usefulness of posterolateral fusion of lumbar spine with allograft bone(tuboplast). J Kor Soc Spine surg. 1998; 5:198–204.17. Lexer E. The History of Bone Graft. Ciln Orthop. 1998; 226:292–8.18. Kurz LT, Garfin SR, Booth RE Jr. Harvesting autogenous iliac bone graft. A review of complication and techniques. Spine (Phila Pa 1976). 1989; 14:1324–31.19. Burwell RG. Studies in the transplantation of bone, V the capacity of fresh and treated homograft of bone to evoke transplantation immunity. J Bone Joint Surg. 1963; 45:386–401.20. Bonfiglio M, Jetter WS. Immunological response to bone. Clin Orthop. 1972; 87:19–27.21. Nandi SK, Roy S, Mukherjee P, Kundu B, De DK, Basu D. Orthopaedic applications of bone graft & graft substitutues: a review. Indian J Med Res. 2010; 132:15–30.22. Rihn JA, Kirkpatrick K, Albert TJ. Graft options in posterolateral and posterior interbody lumbar fusion. Spine (Phila Pa 1976). 2010; 35:1629–39.
Article23. Delecrin J, Takahashi S, Gouin F, Passuti N. A synthetic porous ceramic as a bone graft substitute in the surgical management of scoliosis: a prospective, randomized study. Spine (Phila Pa 1976). 2001; 25:563–9.24. Meadows GR. Adjunctive use of ultra-porous beta-tricalcium phosphate bone void filler in spinal arthrodesis. Orthopedics. 2002; 25(Suppl):579–84.
Article25. Muschik M, Ludwig R, Halbhubner S, Bursche K, Stoll T. Beta tricalcium phosphate as a bone substitute for dorsal spinal fusion in adolescent idiopathic scoliosis: preliminary results of a prospective clinical study. Eur spine J. 2001; 10:178–84.26. Yang JY, Lee JK, Kim DH, et al. The effect of β-tricalcium phosphate in lumbar posterolateral fusion surgery – A prospective study -. J Kor Musculoskelet Transplant Soc. 2006; 6:13–8.27. Knapp DR, Jones ET. Use of cortical cancellous allograft for posterior spinal fusion. Clin Orthop. 1988; 229:99–106.
Article28. Kim JW, Ko YC, Jung CY, Eun IS, Kim OG, Kim CK. Union rates of local autobone and femoral head allobone mixed graft in lumbar posterolateral fusion. J Kor Musculoskelet Transplant Soc. 2010; 10:50–8.29. Zhang M, Wang K, Shi Z, Yang H, Dang X, Wang W. Os-teogenesis of the construct combined BMCs with β-TCP in rat. J plast Reconstr Aesthet Surg. 2010; 63:227–32.30. Orii H. Sotome S, Chen J, Wang J, Shinomiya K. Beta-tricalcium phosphate(beta-TCP) graft combined with bone marrow stromal cells(MSCs) for posterolateral spine fusion. J Med Dent Sci. 2005; 52:51–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of the Bone Union Rates Using a Local Autobone and Bone Graft Substitute Mixed Graft in Lumbar Posterolateral Fusion
- Biphasic Calcium Phosphate and Local Autobone Mixed Graft in Lumbar Posterolateral Fusion
- Union Rates of Autologous Bone Marrow, Local Autobone and Biphasic Calcium Phosphate Mixed Graft in Lumbar Posterolateral Fusion
- Comparison Between Allograft Mixed with Local Bone and Autograft in Posterolateral Lumbar Fusion
- Comparison of the Result of Lumbar Posterolateral Bone Fusion using Autogenous Bone Graft with Allobone Graft