Tuberc Respir Dis.
2016 Jan;79(1):22-30. 10.4046/trd.2016.79.1.22.
Outcome of Inhaler Withdrawal in Patients Receiving Triple Therapy for COPD
- Affiliations
-
- 1Department of Internal Medicinem, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- 3Division of Pulmonology, Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Guri, Korea.
- 4Department of Internal Medicine, Kangwon National University College of Medicine, Chuncheon, Korea.
- 5Department of Internal Medicine, Ewha Womens University Mokdong Hospital, Ewha Womens University School of Medicine, Seoul, Korea.
- 6Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 7Department of Clinical Epidemiology and Biostatistics, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 8Department of Pulmonary and Critical Care Medicine, Asthma Center, and Clinical Research Center for Chronic Obstructive Airway Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sdlee@amc.seoul.kr
- KMID: 2320752
- DOI: http://doi.org/10.4046/trd.2016.79.1.22
Abstract
- BACKGROUND
The purpose of this study was to document outcomes following withdrawal of a single inhaler (step-down) in chronic obstructive pulmonary disease (COPD) patients on triple therapy (long-acting muscarinic antagonist and a combination of long-acting beta2-agonists and inhaled corticosteroid), which a common treatment strategy in clinical practice.
METHODS
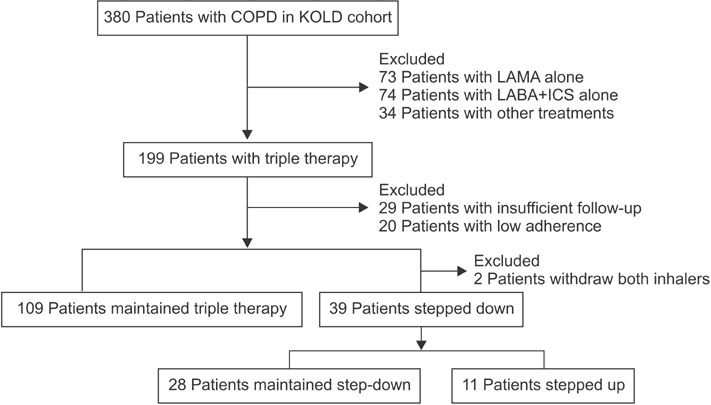
Through a retrospective observational study, COPD patients receiving triple therapy over 2 years (triple group; n=109) were compared with those who had undergone triple therapy for at least 1 year and subsequently, over 9 months, initiated inhaler withdrawal (step-down group, n=39). The index time was defined as the time of withdrawal in the stepdown group and as 1 year after the start of triple therapy in the triple group.
RESULTS
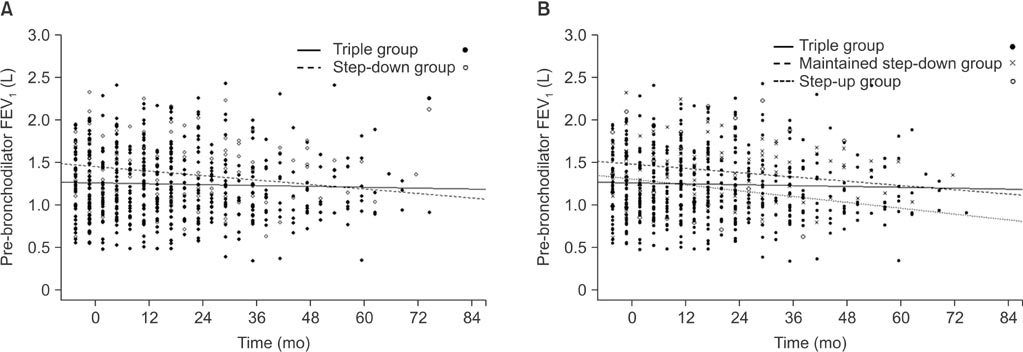
Lung function at the index time was superior and the previous exacerbation frequency was lower in the stepdown group than in the triple group. Step-down resulted in aggravating disease symptoms, a reduced overall quality of life, decreasing exercise performance, and accelerated forced expiratory volume in 1 second (FEV1) decline (54.7+/-15.7 mL/yr vs. 10.7+/-7.1 mL/yr, p=0.007), but there was no observed increase in the frequency of exacerbations.
CONCLUSION
Withdrawal of a single inhaler during triple therapy in COPD patients should be conducted with caution as it may impair the exercise capacity and quality of life while accelerating FEV1 decline.
Keyword
MeSH Terms
Figure
Reference
-
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD [Internet]. Bethesda: National Institutes of Health, GOLD;c2014. [cited 2014 Dec 1]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html.2. National Clinical Guideline Centre. Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care [Internet]. London: National Clinical Guideline Centre;c2010. [cited 2010 Jun 1]. Available from: http://guidance.nice.org.uk/CG101/Guidance/pdf/English.3. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011; 155:179–191.4. van Noord JA, Aumann JL, Janssens E, Verhaert J, Smeets JJ, Mueller A, et al. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest. 2006; 129:509–517.5. van Noord JA, Aumann JL, Janssens E, Smeets JJ, Zaagsma J, Mueller A, et al. Combining tiotropium and salmeterol in COPD: effects on airflow obstruction and symptoms. Respir Med. 2010; 104:995–1004.6. Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009; 6:17–25.7. Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003; 361:449–456.8. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.9. Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002; 166:1084–1091.10. Kim WJ, Oh YM, Sung J, Kim TH, Huh JW, Jung H, et al. Lung function response to 12-week treatment with combined inhalation of long-acting beta2 agonist and glucocorticoid according to ADRB2 polymorphism in patients with chronic obstructive pulmonary disease. Lung. 2008; 186:381–386.11. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173:676–682.12. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007; 370:786–796.13. Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of "triple" therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008; 63:592–598.14. Cazzola M, Ando F, Santus P, Ruggeri P, Di Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007; 20:556–561.15. Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007; 146:545–555.16. Karner C, Cates CJ. Combination inhaled steroid and long-acting beta(2)-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011; (3):CD008532.17. Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014; 3:CD010844.18. Barnes PJ. Inhaled corticosteroids in COPD: a controversy. Respiration. 2010; 80:89–95.19. Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med. 2009; 169:219–229.20. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med. 2010; 123:1001–1006.21. Singh S, Loke YK. An overview of the benefits and drawbacks of inhaled corticosteroids in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010; 5:189–195.22. Wouters EF, Postma DS, Fokkens B, Hop WC, Prins J, Kuipers AF, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax. 2005; 60:480–487.23. van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med. 2002; 166:1358–1363.24. Nadeem NJ, Taylor SJ, Eldridge SM. Withdrawal of inhaled corticosteroids in individuals with COPD: a systematic review and comment on trial methodology. Respir Res. 2011; 12:107.25. Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest. 2010; 137:318–325.26. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554.27. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012; 7:95–99.28. Calverley PM, Spencer S, Willits L, Burge PS, Jones PW; IOSLDE Study Group. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest. 2003; 124:1350–1356.29. Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.30. Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014; 371:1285–1294.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Outcome of Inhaler Withdrawal in Patients Receiving Triple Therapy for COPD
- Assessment of Inhaler Satisfaction and Determinants of High Satisfaction Among Korean COPD Patients
- Recent advance in inhaler medications for chronic obstructive pulmonary disease patients
- Inhalation medications in chronic airway disease
- Diagnosis and treatment of COPD