Tuberc Respir Dis.
2013 Sep;75(3):104-110.
Plasma Osteopontin Is a Useful Diagnostic Biomarker for Advanced Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, Kangwon National University School of Medicine, Chuncheon, Korea. pulmo2@kangwon.ac.kr
- 2Department of Otolaryngology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3The Research Department, Kangwon Regional Cancer Center, Kangwon National University Hospital, Chuncheon, Korea.
- 4The Clinical Research Institute of Kangwon National University Hospital, Chuncheon, Korea.
Abstract
- BACKGROUND
Osteopontin (OPN) and carbonic anhydrase IX (CAIX), which are expressed on the surface of tumor cells, are associated with hypoxia during tumor development and progression. However, the roles of these proteins in the plasma of patients with non-small cell lung cancer (NSCLC) are poorly understood. Herein, we hypothesized that plasma OPN and CAIX levels could be used as diagnostic and prognostic tumor markers in patients with NSCLC.
METHODS
Fifty-three patients with NSCLC and 50 healthy control subjects were enrolled. We selected controls without malignancy and matched them with NSCLC patient cases according to age and gender. Blood samples were collected at the time of diagnosis; the plasma levels of OPN and CAIX were measured by enzyme-linked immunosorbent assays.
RESULTS
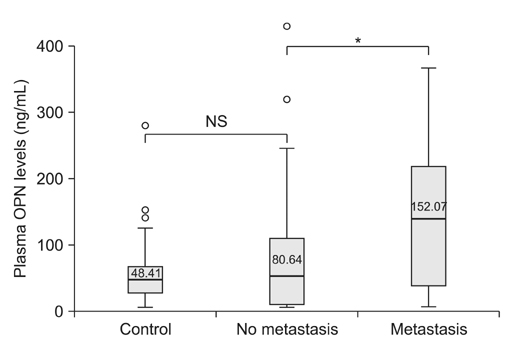
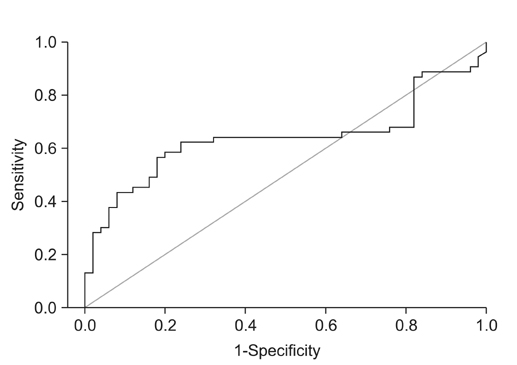
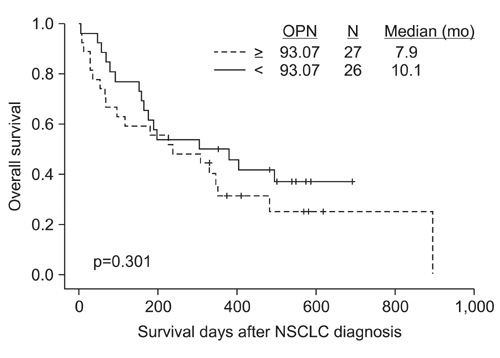
The plasma levels of OPN in the patients with NSCLC were significantly elevated as compared to those in the controls (p=0.016). However, there was no difference in the plasma level of CAIX between the NSCLC patients and controls. NSCLC patients with a distant metastasis had a remarkable increase in plasma OPN compared with patients without metastasis (p=0.026), but no such correlation was found for CAIX. There was no difference in overall survival rates according to the plasma level of OPN between the two groups (by Kaplan-Meier survival analysis).
CONCLUSION
Plasma OPN levels were elevated in patients with NSCLC as compared with the controls, with greater elevation of OPN levels in the advanced stages of disease. Therefore, plasma OPN may have utility as a diagnostic, but not prognostic, biomarker of advanced NSCLC.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.2. Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998; 58:1408–1416.3. Le QT, Chen E, Salim A, Cao H, Kong CS, Whyte R, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006; 12:1507–1514.4. Rittling SR, Chambers AF. Role of osteopontin in tumour progression. Br J Cancer. 2004; 90:1877–1881.5. Weber GF, Lett GS, Haubein NC. Categorical meta-analysis of Osteopontin as a clinical cancer marker. Oncol Rep. 2011; 25:433–441.6. Hu Z, Lin D, Yuan J, Xiao T, Zhang H, Sun W, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non-small cell lung cancer. Clin Cancer Res. 2005; 11:4646–4652.7. Zhao B, Sun T, Meng F, Qu A, Li C, Shen H, et al. Osteopontin as a potential biomarker of proliferation and invasiveness for lung cancer. J Cancer Res Clin Oncol. 2011; 137:1061–1070.8. Bramwell VH, Doig GS, Tuck AB, Wilson SM, Tonkin KS, Tomiak A, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006; 12(11 Pt 1):3337–3343.9. Mrochem J, Sodowski K, Deja R, Walaszek-Gruszka A, Wojcieszek A, Kolosza Z, et al. Evaluation of selected serum protein markers as early detectors of ovarian cancer. Ginekol Pol. 2008; 79:271–275.10. Abu El, Abdel-Aleem A, Ali A, Saber R, Shatat M, Rahem DA, et al. Diagnostic significance of plasma osteopontin in hepatitis C virus-related hepatocellular carcinoma. Ann Hepatol. 2011; 10:296–305.11. Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN Jr, et al. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol. 2008; 26:4771–4776.12. Isa S, Kawaguchi T, Teramukai S, Minato K, Ohsaki Y, Shibata K, et al. Serum osteopontin levels are highly prognostic for survival in advanced non-small cell lung cancer: results from JMTO LC 0004. J Thorac Oncol. 2009; 4:1104–1110.13. Blasberg JD, Pass HI, Goparaju CM, Flores RM, Lee S, Donington JS. Reduction of elevated plasma osteopontin levels with resection of non-small-cell lung cancer. J Clin Oncol. 2010; 28:936–941.14. Winum JY, Scozzafava A, Montero JL, Supuran CT. Inhibition of carbonic anhydrase IX: a new strategy against cancer. Anticancer Agents Med Chem. 2009; 9:693–702.15. Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem. 2001; 9:703–714.16. Kim SJ, Rabbani ZN, Vollmer RT, Schreiber EG, Oosterwijk E, Dewhirst MW, et al. Carbonic anhydrase IX in early-stage non-small cell lung cancer. Clin Cancer Res. 2004; 10:7925–7933.17. Pena C, Lathia C, Shan M, Escudier B, Bukowski RM. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res. 2010; 16:4853–4863.18. Hyrsl L, Zavada J, Zavadova Z, Kawaciuk I, Vesely S, Skapa P. Soluble form of carbonic anhydrase IX (CAIX) in transitional cell carcinoma of urinary tract. Neoplasma. 2009; 56:298–302.19. Muller V, Riethdorf S, Rack B, Janni W, Fasching PA, Solomayer E, et al. Prospective evaluation of serum tissue inhibitor of metalloproteinase 1 and carbonic anhydrase IX in correlation to circulating tumor cells in patients with metastatic breast cancer. Breast Cancer Res. 2011; 13:R71.20. Ilie M, Mazure NM, Hofman V, Ammadi RE, Ortholan C, Bonnetaud C, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer. 2010; 102:1627–1635.21. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007; 2:706–714.22. Ahmed M, Behera R, Chakraborty G, Jain S, Kumar V, Sharma P, et al. Osteopontin: a potentially important therapeutic target in cancer. Expert Opin Ther Targets. 2011; 15:1113–1126.23. Chakraborty G, Jain S, Behera R, Ahmed M, Sharma P, Kumar V, et al. The multifaceted roles of osteopontin in cell signaling, tumor progression and angiogenesis. Curr Mol Med. 2006; 6:819–830.24. Chang YS, Kim HJ, Chang J, Ahn CM, Kim SK, Kim SK. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007; 57:373–380.25. Wagner PD, Srivastava S. New paradigms in translational science research in cancer biomarkers. Transl Res. 2012; 159:343–353.26. Prasse A, Stahl M, Schulz G, Kayser G, Wang L, Ask K, et al. Essential role of osteopontin in smoking-related interstitial lung diseases. Am J Pathol. 2009; 174:1683–1691.27. Hillas G, Loukides S, Kostikas K, Simoes D, Petta V, Konstantellou E, et al. Increased levels of osteopontin in sputum supernatant of smoking asthmatics. Cytokine. 2013; 61:251–255.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Osteopontin on Normal and Malignant Ovarian Epithelial Cell
- Management of Locally Advanced Non-small Cell Lung Cancer
- The Role and Significance of Biomarker for Plasma G-CSF in Patients with Primary Lung Cancer
- Combined Modality Therapy for Locally Advanced Non-Small Cell Lung Cancer
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics