Tuberc Respir Dis.
2013 Jun;74(6):264-268.
The Clinical Assessment of Protease-Activated Receptor-2 Expression in Inflammatory Cells from Peripheral Blood and Bronchoalveolar Lavage Fluid in Idiopathic Pulmonary Fibrosis
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine and Lung Institute, Seoul National University College of Medicine, Seoul, Korea. cgyoo@snu.ac.kr
Abstract
- BACKGROUND
Idiopathic pulmonary fibrosis (IPF) is a lethal pulmonary fibrotic disease. In general, the exaggerated activation of the coagulation cascade has been observed during initiation or maintenance of the fibrotic disease. In our recent study, immunohistochemical expression of protease-activated receptor-2 (PAR-2), which plays a key role in coagulation cascade, was observed in surgical specimen of IPF patients, and associated with poor clinical outcome. The aim of this study was to evaluate the overexpression of PAR-2 in inflammatory cells from peripheral blood and bronchoalveolar lavage fluid in IPF patients.
METHODS
From May 2011 to March 2012, IPF patients and controls were enrolled in Seoul National University Hospital. Peripheral blood and bronchoalveolar lavage fluid were collected for analysis of PAR-2 expression. Flow cytometry and reverse transcription polymerase chain reaction were used for PAR-2 receptor and mRNA assessment.
RESULTS
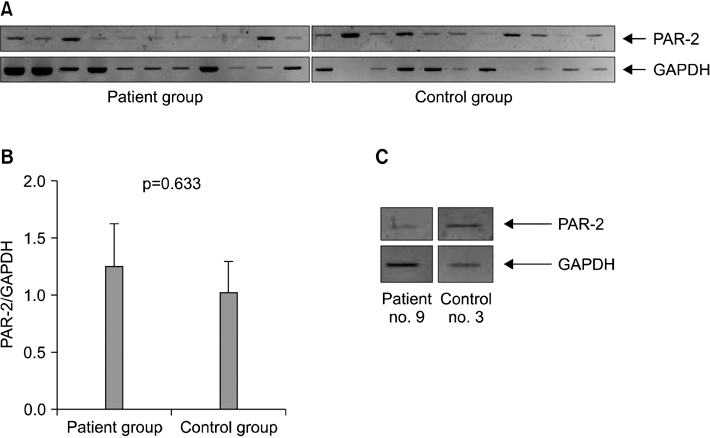
Twelve IPF patients and 14 controls were included in this study. Among them, flow cytometry analysis was conducted from 26 peripheral blood (patient group, 11; control group, 13) and 7 bronchoalveolar lavage fluid (patient group, 5; control group, 2). The expression of PAR-2 receptor was not different between patient and control groups (p=0.074). Among all 24 population, PAR-2 mRNA assessment was performed in 19 persons (patient group, 10; control group, 9). The mRNA expression of PAR-2 was not significant different (p=0.633).
CONCLUSION
In IPF patients, PAR-2 receptor and mRNA expression were not different from control group.
MeSH Terms
Figure
Reference
-
1. American Thoracic Society. European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002; 165:277–304.2. Harari S, Caminati A. Idiopathic pulmonary fibrosis. Allergy. 2005; 60:421–435.3. Caminati A, Harari S. IPF: new insight in diagnosis and prognosis. Respir Med. 2010; 104:Suppl 1. S2–S10.4. Selman M, King TE, Pardo A. American Thoracic Society. European Respiratory Society. American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001; 134:136–151.5. Chambers RC. Procoagulant signalling mechanisms in lung inflammation and fibrosis: novel opportunities for pharmacological intervention? Br J Pharmacol. 2008; 153:Suppl 1. S367–S378.6. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994; 91:9208–9212.7. Nystedt S, Emilsson K, Larsson AK, Strombeck B, Sundelin J. Molecular cloning and functional expression of the gene encoding the human proteinase-activated receptor 2. Eur J Biochem. 1995; 232:84–89.8. D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, et al. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998; 46:157–164.9. Miotto D, Hollenberg MD, Bunnett NW, Papi A, Braccioni F, Boschetto P, et al. Expression of protease activated receptor-2 (PAR-2) in central airways of smokers and non-smokers. Thorax. 2002; 57:146–151.10. Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001; 108:797–803.11. Peters T, Henry PJ. Protease-activated receptors and prostaglandins in inflammatory lung disease. Br J Pharmacol. 2009; 158:1017–1033.12. Borensztajn K, Bresser P, van der Loos C, Bot I, van den Blink B, den Bakker MA, et al. Protease-activated receptor-2 induces myofibroblast differentiation and tissue factor up-regulation during bleomycin-induced lung injury: potential role in pulmonary fibrosis. Am J Pathol. 2010; 177:2753–2764.13. Wygrecka M, Kwapiszewska G, Jablonska E, von Gerlach S, Henneke I, Zakrzewicz D, et al. Role of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011; 183:1703–1714.14. Park YS, Park CM, Lee HJ, Goo JM, Chung DH, Lee SM, et al. Clinical implication of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Respir Med. 2013; 107:256–262.15. Hernandez-Rodriguez NA, Cambrey AD, Harrison NK, Chambers RC, Gray AJ, Southcott AM, et al. Role of thrombin in pulmonary fibrosis. Lancet. 1995; 346:1071–1073.16. Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997; 156(2 Pt 1):631–636.17. Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia: comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000; 161(2 Pt 1):454–462.18. Noth I, Anstrom KJ, Calvert SB, de Andrade J, Flaherty KR, Glazer C, et al. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012; 186:88–95.19. Lewkowich IP, Day SB, Ledford JR, Zhou P, Dienger K, Wills-Karp M, et al. Protease-activated receptor 2 activation of myeloid dendritic cells regulates allergic airway inflammation. Respir Res. 2011; 12:122.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytologic Findings of Bronchoalveolar Lavage
- Child with Urinary Tract Infection
- The Diagnostic Value of Bronchoalveolar lavage fluid microscopic study and PCR in Pulmonary tuberculosis
- Changes of the cellularities in the bronchoalveolar lavage fluid of the experimental silicosis
- Deficiency of Sphingosine-1-Phosphate Receptor 2 (S1Pâ‚‚) Attenuates Bleomycin-Induced Pulmonary Fibrosis