Tuberc Respir Dis.
2011 Jun;70(6):482-489.
Effect of Apocynin on Acute Lung Injury in Rats Given Interleukin-1alpha Intratracheally
- Affiliations
-
- 1Department of Physiology, The Catholic University of Daegu School of Medicine, Daegu, Korea. leeym@cu.ac.kr
Abstract
- BACKGROUND
Based on the assertion that apocynin diminishes acute lung injury (ALI) by inhibition of NADPH oxidase, the effect of apocynin was tested in interleukin-1alpha (IL-1)-induced ALI in rats.
METHODS
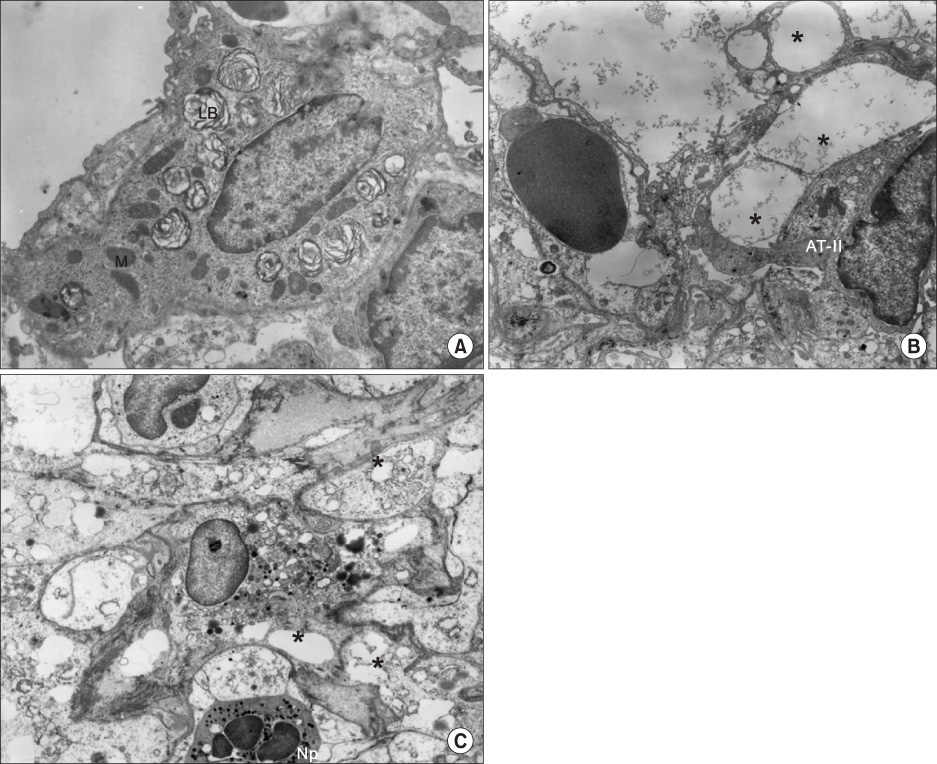
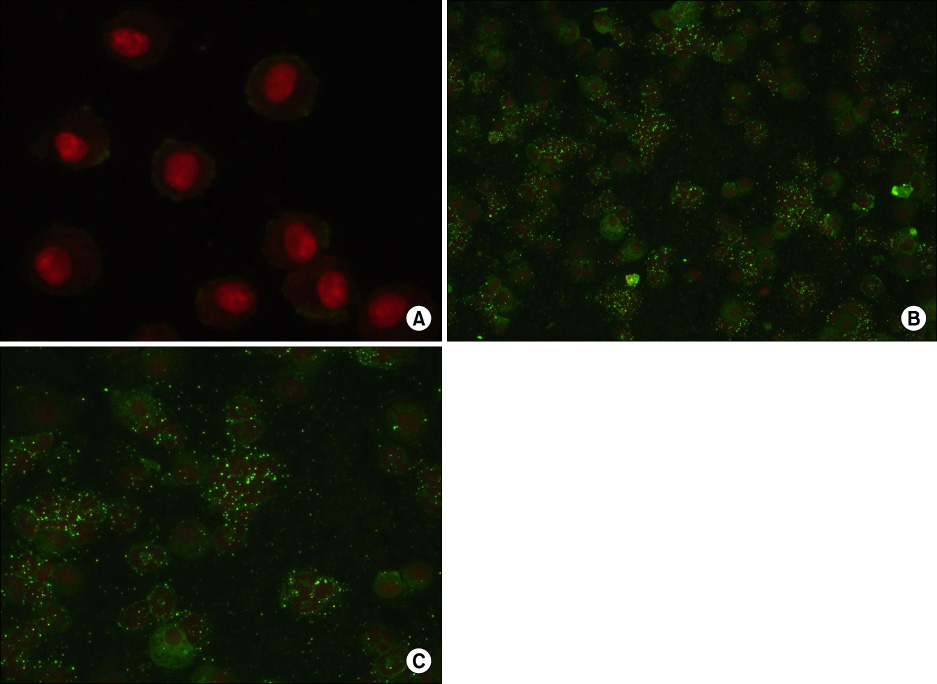
IL-1 was insufflated into the trachea of Sprague-Dawley rats to induce ALI, and apocynin (8 mg/kg) was given intravenously for inhibition of NADPH oxidase. In addition, we determined whether apocynin inhibited generation of superoxide anions from isolated human neutrophils. Five hours after IL-1 instillation, lung injury parameters, expression of cytosolic phospholipase A2 (cPLA2) by cells from bronchoalveolar lavage (BAL), an index of oxidative stress in lung tissues (gamma-glutamyltranspeptidase, activity), and ultrastructure of alveolar type II (AT II) cells were evaluated.
RESULTS
Apocynin decreased the generation of free radicals from phorbol myristate (PMA)-activated neutrophils in vitro, but did not ameliorate ALI. IL-1 induced enhancement of the expression of cPLA2 on neutrophils was not altered by apocynin.
CONCLUSION
Apocynin induced suppression of the generation of superoxide anions from neutrophils by inhibition of NADPH oxidase does not attenuate IL-1-induced ALI in rats.
Keyword
MeSH Terms
-
Acetophenones
Acute Lung Injury
Animals
Bronchoalveolar Lavage
Cytosol
Free Radicals
Humans
Interleukin-1
Interleukin-1alpha
Lung
Lung Injury
Myristic Acid
NADPH Oxidase
Neutrophils
Oxidative Stress
Phorbols
Phospholipases A2
Rats
Rats, Sprague-Dawley
Superoxides
Trachea
Acetophenones
Free Radicals
Interleukin-1
Interleukin-1alpha
Myristic Acid
NADPH Oxidase
Phorbols
Phospholipases A2
Superoxides
Figure
Reference
-
1. Downey GP, Granton JT. Mechanisms of acute lung injury. Curr Opin Pulm Med. 1997. 3:234–241.2. Newton R, Kuitert LM, Slater DM, Adcock IM, Barnes PJ. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci. 1997. 60:67–78.3. Pruzanski W, Vadas P. Phospholipase A2--a mediator between proximal and distal effectors of inflammation. Immunol Today. 1991. 12:143–146.4. Kitsiouli E, Nakos G, Lekka ME. Phospholipase A2 subclasses in acute respiratory distress syndrome. Biochim Biophys Acta. 2009. 1792:941–953.5. Sun K, Qu X, Gao L, Myatt L. Dexamethasone fails to inhibit the induction of cytosolic phospholipase A(2) expression by interleukin-1beta in cultured primary human amnion fibroblasts. Placenta. 2006. 27:164–170.6. Dana R, Malech HL, Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994. 297:217–223.7. Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, et al. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 2011. 81:636–648.8. Yang Z, Sharma AK, Marshall M, Kron IL, Laubach VE. NADPH oxidase in bone marrow-derived cells mediates pulmonary ischemia-reperfusion injury. Am J Respir Cell Mol Biol. 2009. 40:375–381.9. Vejrazka M, Mícek R, Stípek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005. 1722:143–147.10. Shmelzer Z, Haddad N, Admon E, Pessach I, Leto TL, Eitan-Hazan Z, et al. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J Cell Biol. 2003. 162:683–692.11. Wong RK, Pettit AI, Quinn PA, Jennings SC, Davies JE, Ng LL. Advanced glycation end products stimulate an enhanced neutrophil respiratory burst mediated through the activation of cytosolic phospholipase A2 and generation of arachidonic acid. Circulation. 2003. 108:1858–1864.12. Rubin BB, Downey GP, Koh A, Degousee N, Ghomashchi F, Nallan L, et al. Cytosolic phospholipase A2-alpha is necessary for platelet-activating factor biosynthesis, efficient neutrophil-mediated bacterial killing, and the innate immune response to pulmonary infection: cPLA2-alpha does not regulate neutrophil NADPH oxidase activity. J Biol Chem. 2005. 280:7519–7529.13. Hybertson BM, Lee YM, Cho HG, Cho OJ, Repine JE. Alveolar type II cell abnormalities and peroxide formation in lungs of rats given IL-1 intratracheally. Inflammation. 2000. 24:289–303.14. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989. 180:136–139.15. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol. 1985. 59:1978–1985.16. Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation states: therapeutic challenges. Shock. 1995. 3:157–166.17. Haslett C, Guthrie LA, Kopaniak MM, Johnston RB Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985. 119:101–110.18. Meister A, Tate SS, Griffith OW. Gamma-glutamyl transpeptidase. Methods Enzymol. 1981. 77:237–253.19. Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling--regulation of the immune response in normal and critically ill states. Crit Care Med. 2000. 28:4 Suppl. N3–N12.20. Paterniti I, Galuppo M, Mazzon E, Impellizzeri D, Esposito E, Bramanti P, et al. Protective effects of apocynin, an inhibitor of NADPH oxidase activity, in splanchnic artery occlusion and reperfusion. J Leukoc Biol. 2010. 88:993–1003.21. Pearse DB, Wagner EM, Sylvester JT. Edema clearance in isolated sheep lungs. J Appl Physiol. 1993. 74:126–132.22. Dodd-o JM, Welsh LE, Salazar JD, Walinsky PL, Peck EA, Shake JG, et al. Effect of NADPH oxidase inhibition on cardiopulmonary bypass-induced lung injury. Am J Physiol Heart Circ Physiol. 2004. 287:H927–H936.23. Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990. 8:251–258.24. Mårtensson J, Jain A, Stole E, Frayer W, Auld PA, Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci USA. 1991. 88:9360–9364.25. Stolk J, Rossie W, Dijkman JH. Apocynin improves the efficacy of secretory leukocyte protease inhibitor in experimental emphysema. Am J Respir Crit Care Med. 1994. 150:1628–1631.26. Wang W, Suzuki Y, Tanigaki T, Rank DR, Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med. 1994. 150:1449–1452.27. Pearse DB, Dodd JM. Ischemia-reperfusion lung injury is prevented by apocynin, a novel inhibitor of leukocyte NADPH oxidase. Chest. 1999. 116:1 Suppl. 55S–56S.28. Lee YM, Hybertson BM, Terada LS, Repine AJ, Cho HG, Repine JE. Mepacrine decreases lung leak in rats given interleukin-1 intratracheally. Am J Respir Crit Care Med. 1997. 155:1624–1628.29. Linkous A, Yazlovitskaya E. Cytosolic phospholipase A2 as a mediator of disease pathogenesis. Cell Microbiol. 2010. 12:1369–1377.30. Bréchard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. J Leukoc Biol. 2008. 84:1223–1237.31. Kim C, Kim JY, Kim JH. Cytosolic phospholipase A(2), lipoxygenase metabolites, and reactive oxygen species. BMB Rep. 2008. 41:555–559.32. O'Dowd YM, El-Benna J, Perianin A, Newsholme P. Inhibition of formyl-methionyl-leucyl-phenylalanine-stimulated respiratory burst in human neutrophils by adrenaline: inhibition of Phospholipase A2 activity but not p47phox phosphorylation and translocation. Biochem Pharmacol. 2004. 67:183–190.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Neutrophil Elastase inhibitor, ICI 200,355, on Interleukin-1 Induced acute lung injury in rats
- The study for the roles of intratracheally administered histamine in the neutrophil-mediated acute lung injury in rats:
- Effect of the inhibition of platelet activating factor on oxidative lung injury induced by interleukin-1 alpha
- The Effect of Heat Shock Response on the Tumor Necrosis Factor-alpha-induced Acute Lung Injury in Rats
- The Effect of Interleukin-1alpha on Trabecular Outflow Resistance in Rat Eyes