Pediatr Gastroenterol Hepatol Nutr.
2015 Jun;18(2):144-148. 10.5223/pghn.2015.18.2.144.
Atypical beta-Catenin Activated Child Hepatocellular Tumor
- Affiliations
-
- 1Department of Radiology, Diskapi Yildirim Beyazit Training and Research Hospital, Ankara, Turkey. aturanrad@gmail.com
- 2Department of Radiology, Ankara Pediatric Hematology and Oncology Training and Research Hospital, Ankara, Turkey.
- 3Department of Patology, Ankara Pediatric Hematology and Oncology Training and Research Hospital, Ankara, Turkey.
- 4Department of Pediatrics, Ankara Pediatric Hematology and Oncology Training and Research Hospital, Ankara, Turkey.
- 5Department of Radiology, Ankara Ataturk Training and Research Hospital, Ankara, Turkey.
- KMID: 2315563
- DOI: http://doi.org/10.5223/pghn.2015.18.2.144
Abstract
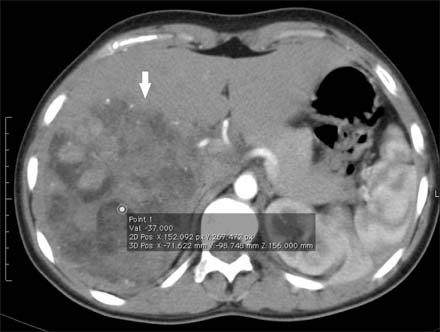
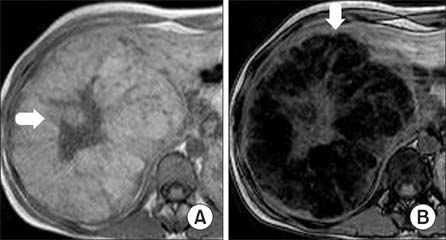
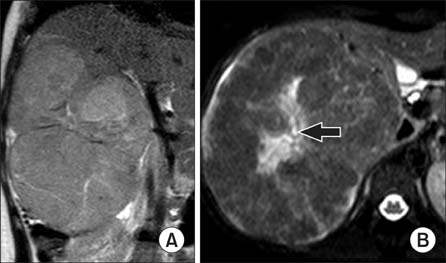
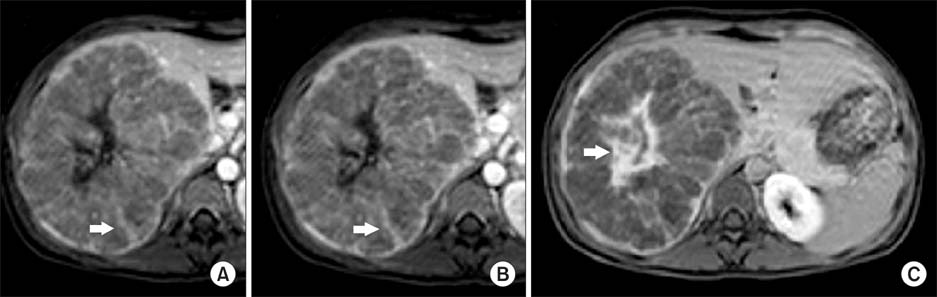
- Hepatocellular adenomas are a benign, focal, hepatic neoplasm that have been divided into four subtypes according to the genetic and pathological features. The beta-catenin activated subtype accounts for 10-15% of all hepatocellular adenomas and specific magnetic resonance imaging features have been defined for different hepatocellular adenomas subtypes. The current study aimed to report the magnetic resonance imaging features of a well differentiated hepatocellular carcinoma that developed on the basis of beta-catenin activated hepatocellular adenomas in a child. In this case, atypical diffuse steatosis was determined in the lesion. In the literature, diffuse steatosis, which is defined as a feature of the hepatocyte nuclear factor-1alpha-inactivated hepatocellular adenomas subtype, has not been previously reported in any beta-catenin activated hepatocellular adenomas case. Interlacing magnetic resonance imaging findings between subtypes show that there are still many mysteries about this topic and larger studies are warranted.
MeSH Terms
Figure
Reference
-
1. Chung EM, Cube R, Lewis RB, Conran RM. From the archives of the AFIP: pediatric liver masses: radiologic-pathologic correlation part 1. Benign tumors. Radiographics. 2010; 30:801–826.
Article2. van Aalten SM, Thomeer MG, Terkivatan T, Dwarkasing RS, Verheij J, de Man RA, et al. Hepatocellular adenomas: correlation of MR imaging findings with pathologic subtype classification. Radiology. 2011; 261:172–181.
Article3. Laumonier H, Bioulac-Sage P, Laurent C, Zucman-Rossi J, Balabaud C, Trillaud H. Hepatocellular adenomas: magnetic resonance imaging features as a function of molecular pathological classification. Hepatology. 2008; 48:808–818.
Article4. Yoneda N, Matsui O, Kitao A, Kozaka K, Gabata T, Sasaki M, et al. Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol. 2012; 30:777–782.
Article5. Chu HH, Moon WS. β-catenin activated hepatocellular adenoma. Clin Mol Hepatol. 2013; 19:185–189.
Article6. Manichon AF, Bancel B, Durieux-Millon M, Ducerf C, Mabrut JY, Lepogam MA, et al. Hepatocellular adenoma: evaluation with contrast-enhanced ultrasound and MRI and correlation with pathologic and phenotypic classification in 26 lesions. HPB Surg. 2012; 2012:418745.
Article7. Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: implications for imaging and management. Radiology. 2011; 258:673–693.
Article8. Katabathina VS, Menias CO, Shanbhogue AK, Jagirdar J, Paspulati RM, Prasad SR. Genetics and imaging of hepatocellular adenomas: 2011 update. Radiographics. 2011; 31:1529–1543.
Article9. Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006; 43:515–524.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta-catenin activated hepatocellular adenoma
- beta-Catenin Activated Hepatocellular Adenoma: A Report of Three Cases in Korea
- Expression of beta-catenin in Hepatocellular Carcinoma in Relation to Tumor Cell Proliferation and Cyclin D1 Expression
- Immunohistochemical Study of beta-catenin Expression between Hepatocellular Carcinoma and Cholangiocarcinoma
- Correlation of the Nuclear beta-catenin Expression with the Clinicopathological Parameters of Hepatocellular Carcinoma