Korean J Urol.
2011 Jul;52(7):494-497.
Limited Expression of Cytochrome P450 17alpha-Hydroxylase/17,20-Lyase in Prostate Cancer Cell Lines
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea. skhong@snubh.org
Abstract
- PURPOSE
Cytochrome P450 17alpha-hydroxylase/17,20-lyase (CYP17A1) is a key enzyme in the androgen biosynthesis pathway. CYP17A1 has been focused on because of the promising results of a potent CYP17A1 inhibitor in the treatment of castration-resistant prostate cancer (CRPC). A hypothesis that intratumoral androgenesis may play a role in the progression of CRPC has recently been postulated. Thus, we evaluated whether commonly used prostate cancer cell lines express CYP17A1.
MATERIALS AND METHODS
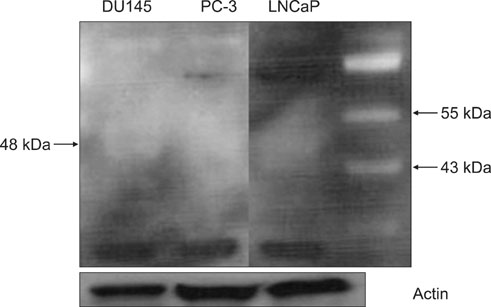
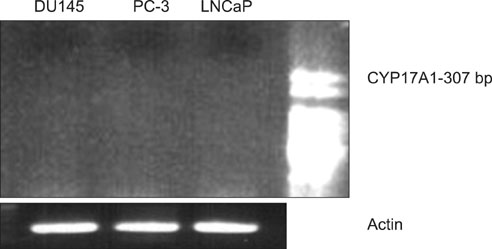
Androgen-sensitive LNCaP and androgen-insensitive PC-3 and DU145 cells were used. To evaluate the expression of CYP17A1 protein and RNA, we performed Western blotting and RT-PCR, respectively.
RESULTS
We were unable to detect either CYP17A1 protein or RNA in any of the cell lines tested. We failed to detect any expression of CYP17A1, despite several repetitions of these techniques under different conditions.
CONCLUSIONS
The expression of CYP17A1 protein and RNA in LNCaP, PC-3, and DU145 cells appears to be either absent or too low for detection. The mechanism of action of abiraterone acetate, a CYP17A1 inhibitor, may be related more to adrenal androgen blockade than to intratumoral androgenesis.
MeSH Terms
Figure
Reference
-
1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009. 59:225–249.2. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009. 41:122–131.3. Song C, Kang T, Lee MS, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in Korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: a multi-institutional update. Korean J Urol. 2007. 48:125–130.4. Goktas S, Crawford ED. Optimal hormonal therapy for advanced prostatic carcinoma. Semin Oncol. 1999. 26:162–173.5. Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991. 324:236–245.6. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004. 351:1502–1512.7. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004. 351:1513–1520.8. Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005. 96:1241–1246.9. Miller WL, Auchus RJ, Geller DH. The regulation of 17,20 lyase activity. Steroids. 1997. 62:133–142.10. Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004. 22:281–288.11. Attard G, Reid AH, A'Hern R, Parker C, Oommen NB, Folkerd E, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009. 27:3742–3748.12. Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008. 26:4563–4571.13. O'Donnell A, Judson I, Dowsett M, Raynaud F, Dearnaley D, Mason M, et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br J Cancer. 2004. 90:2317–2325.14. Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010. 28:1489–1495.15. Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008. 68:6407–6415.16. Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008. 68:4447–4454.17. Rowlands MG, Barrie SE, Chan F, Houghton J, Jarman M, McCague R, et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17,20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysis. J Med Chem. 1995. 38:4191–4197.18. Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997. 57:314–319.19. Gregory CW, Johnson RT Jr, Mohler JL, French FS, Wilson EM. Androgen receptor stabilization in recurrent prostate cancer is associated with hypersensitivity to low androgen. Cancer Res. 2001. 61:2892–2898.20. Mohler JL, Gregory CW, Ford OH 3rd, Kim D, Weaver CM, Petrusz P, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004. 10:440–448.21. Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004. 10:7121–7126.22. Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010. 28:1496–1501.23. De Bono JS, Logothetis CJ, Fizazi K, North S, Chu L, Chi KN, et al. Abiraterone acetate (AA) plus low dose prednisone (P) improves overall survival (OS) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) who have progressed after docetaxel-based chemotherapy (chemo): results of COU-AA-301, a randomized, double-blind, placebo-controlled phase III study. The 35th European Society for Medical Oncology (ESMO) annual meeting 2010. Annals of Oncology. 2010. 21:suppl 8. viii3.24. Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008. 295:115–120.25. Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010. 70:1256–1264.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Six Cases of Congenital Adrenal Hyperplasia That Were Due to 17alpha-hydroxylase/17,20-lyase Deficiency
- Two cases of 17α-hydroxylase/17,20-lyase deficiency caused by the CYP17A1 mutation
- Untreated Congenital Adrenal Hyperplasia with 17-alpha Hydroxylase/17,20-Lyase Deficiency Presenting as Massive Adrenocortical Tumor
- A case of 17 alpha-hydroxylase deficiency
- Steroidogenic electron-transfer factors and their diseases