Korean J Urol.
2012 Apr;53(4):275-279.
Efficacy and Safety of Propiverine in Children with Overactive Bladder
- Affiliations
-
- 1Department of Urology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. minki.baek@samsung.com
- 2Department of Urology, School of Medicine, Kyung Hee University, Seoul, Korea.
- 3Department of Urology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Seoul Samsung Urology Clinic/Gynecology Health Care Center, Ulsan, Korea.
Abstract
- PURPOSE
Antimuscarinic therapy remains one of the most common forms of therapy for overactive bladder (OAB) in children. However, few clinical studies on the outcomes of antimuscarinics in children with OAB have been published. Therefore, we evaluated the efficacy and safety of propiverine, which is frequently prescribed for the treatment of pediatric OAB.
MATERIALS AND METHODS
We retrospectively reviewed children with OAB treated with propiverine within the past 5 years. The response rates were compared between the non-urge incontinence (non-UI) and urge incontinence (UI groups). The cumulative response rate by treatment duration was also compared between the two groups.
RESULTS
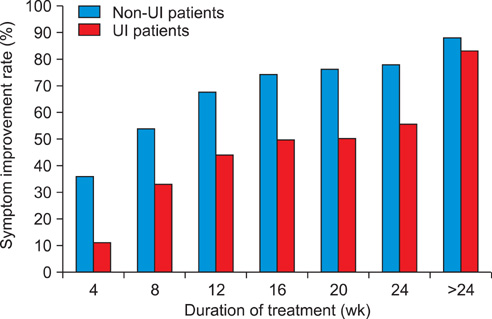
Among a total of 68 children, 50 children (73.5%) experienced UI. The overall response rate was 86.8%. Functional bladder capacity after treatment was 150 ml, which represented an increase compared with the value (140 ml) before treatment. The voiding frequency per day decreased from 14.0 to 8.5 times. The overall response rate (88.0%) in the non-UI group was not significantly different from that seen in the UI group (83.3%; p>0.05). In non-UI children, the cumulative response rates were 36.0%, 54.0%, 68.0%, 74.0%, 76.0%, and 78.0% at 4, 8, 12, 16, 20, and 24 weeks, respectively. The cumulative response rates in the UI children were 11.1%, 33.3%, 44.4%, 50.0%, 50.0%, and 55.6%, respectively during the same respective time periods. Adverse effects were identified in only two (2.9%) patients, and neither case was severe.
CONCLUSIONS
Propiverine is effective and well tolerated as a treatment for children suffering from OAB with or without UI.
Keyword
MeSH Terms
Figure
Reference
-
1. Kwak KW, Park KH. Clinical inconsistency of lower urinary tract symptoms between questionnaire and bladder diary in children with nocturnal enuresis. J Urol. 2008. 180:1085–1089.2. Link CL, Lutfey KE, Steers WD, McKinlay JB. Is abuse causally related to urologic symptoms? Results from the Boston Area Community Health (BACH) Survey. Eur Urol. 2007. 52:397–406.3. Hellström AL, Hanson E, Hansson S, Hjälmås K, Jodal U. Micturition habits and incontinence in 7-year-old Swedish school entrants. Eur J Pediatr. 1990. 149:434–437.4. Kajiwara M, Inoue K, Kato M, Usui A, Kurihara M, Usui T. Nocturnal enuresis and overactive bladder in children: an epidemiological study. Int J Urol. 2006. 13:36–41.5. Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. Prevalence and associated factors of overactive bladder in Korean children 5-13 years old: a nationwide multicenter study. Urology. 2009. 73:63–67.6. Franco I. Overactive bladder in children. Part 1: pathophysiology. J Urol. 2007. 178(3 Pt 1):761–768.7. Saini R, Gonzalez RR, Te AE. Chronic pelvic pain syndrome and the overactive bladder: the inflammatory link. Curr Urol Rep. 2008. 9:314–319.8. Steers WD. Pathophysiology of overactive bladder and urge urinary incontinence. Rev Urol. 2002. 4:Suppl 4. S7–S18.9. Hellström AL. Pathophysiology ochildren with dysfunctional bladder. Scand J Urol Nephrol Suppl. 1992. 141:106–107.10. Diokno AC, Appell RA, Sand PK, Dmochowski RR, Gburek BM, Klimberg IW, et al. Prospective, randomized, double-blind study of the efficacy and tolerability of the extended-release formulations of oxybutynin and tolterodine for overactive bladder: results of the OPERA trial. Mayo Clin Proc. 2003. 78:687–695.11. Madersbacher H, Mürtz G. Efficacy, tolerability and safety profile of propiverine in the treatment of the overactive bladder (non-neurogenic and neurogenic). World J Urol. 2001. 19:324–335.12. Kreder K, Mayne C, Jonas U. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002. 41:588–595.13. Zinner N, Gittelman M, Harris R, Susset J, Kanelos A, Auerbach S, et al. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004. 171(6 Pt 1):2311–2315.14. Chapple CR, Rechberger T, Al-Shukri S, Meffan P, Everaert K, Huang M, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004. 93:303–310.15. Hoebeke P, De Pooter J, De Caestecker K, Raes A, Dehoorne J, Van Laecke E, et al. Solifenacin for therapy resistant overactive bladder. J Urol. 2009. 182:4 Suppl. 2040–2044.16. Um JM, Kim KM. Efficacy and tolerability of extended-release oxybutynin in children with a neurogenic bladder. Korean J Urol. 2007. 48:1064–1068.17. Alloussi S, Mürtz G, Braun R, Gerhardt U, Heinrich M, Hellmis E, et al. Efficacy, tolerability and safety of propiverine hydrochloride in comparison to oxybutynin in children with urge incontinence due to overactive bladder: results of a multicentre observational cohort study. BJU Int. 2010. 106:550–556.18. Marschall-Kehrel D, Feustel C, Persson de Geeter C, Stehr M, Radmayr C, Sillén U, et al. Treatment with propiverine in children suffering from nonneurogenic overactive bladder and urinary incontinence: results of a randomized placebo-controlled phase 3 clinical trial. Eur Urol. 2009. 55:729–736.19. Wada Y, Yoshida M, Kitani K, Kikukawa H, Ichinose A, Takahashi W, et al. Comparison of the effects of various anticholinergic drugs on human isolated urinary bladder. Arch Int Pharmacodyn Ther. 1995. 330:76–89.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of Propiverine Hydrochloride 40mg in Treatment of Overactive Bladder : Prospective, Multicenter, Observational study
- The Efficacy and Safety of Combination Therapy with Alpha-Blocker and Low-Dose Propiverine Hydrochloride for Benign Prostatic Hyperplasia Accompanied by Overactive Bladder Symptoms
- Review of the Anticholinergics for the Treatment of Overactive Bladder: 2009 Update
- The Effects of Combined Therapy with Propiverine (BUP-4(R)) after alpha-blocker on the Improvements of Quality of Life Scores in Patients with Benign Prostatic Hyperplasia Accompaniedwith Overactive Bladder
- Urodynamic Effects of Propiverine on Detrusor Overactivity and Abdominal Straining during Voiding in Awake Rats with Intravesical Prostaglandin E2 Instillation