Korean J Urol.
2009 Oct;50(10):947-954.
The Effect of Finasteride on Microvessel Density and Expression of Vascular Endothelial Growth Factor and 5alpha-Reductase in Prostatic Hyperplasia
- Affiliations
-
- 1Department of Urology, College of Medicine, Konyang University, Daejeon, Korea. ovalboy@hanmail.net
Abstract
- PURPOSE
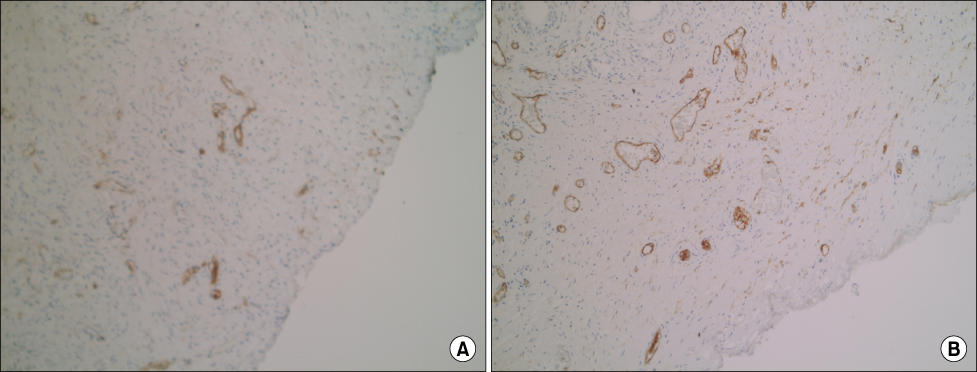
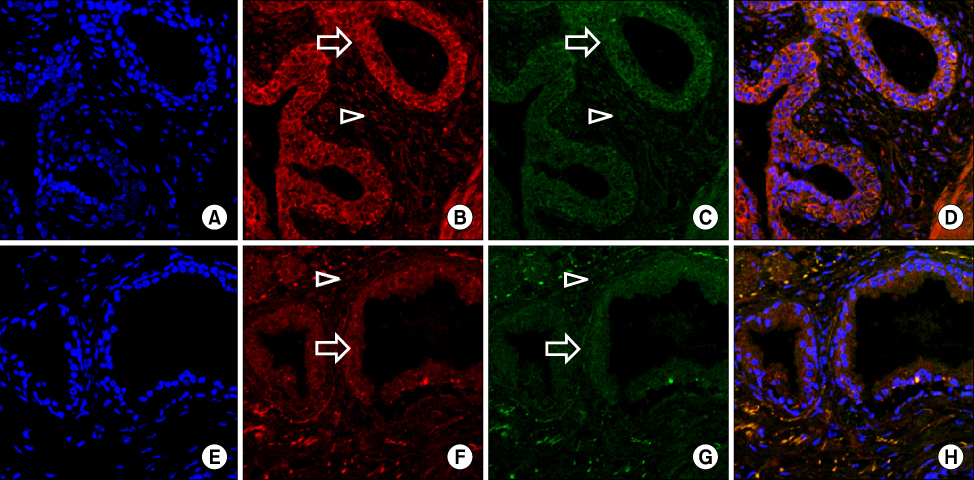
Vascular endothelial growth factor (VEGF), a potent stimulator of angiogenesis and microvessel density (MVD), which is an important indicator of neoangiogenesis, were independently evaluated to elucidate the mechanism of decreased bleeding observed in patients treated with finasteride, an inhibitor of 5alpha-reductase (5AR). We evaluated MVD and the expression of VEGF and 5AR type II in patients with benign prostatic hyperplasia (BPH) treated with finasteride. MATERIALS AND METHODS: The study included 61 patients undergoing transurethral prostatectomy (TURP) for BPH. Among these patients, 29 had well-preserved paraffin blocks, 13 of whom were given finasteride for a minimum of 3 weeks before surgery; the remaining 16 patients served as controls. MVD was calculated by counting the number of positively stained blood vessels on 5 random, high-power fields within the prostatic section. Expressions of VEGF and 5AR type II were analyzed with a confocal laser scanning microscope and an image analyzer.
RESULTS
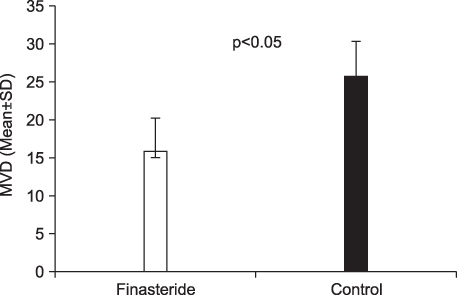
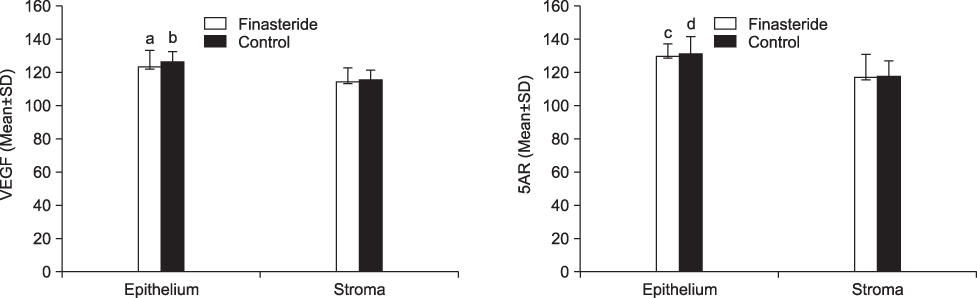
Prostatic MVD was significantly lower in the finasteride-treated group (p<0.05). The expression of VEGF and 5AR type II at the level of the prostatic glandular epithelium and stroma was not significantly different between the 2 groups. VEGF and 5AR type II were more strongly expressed in the epithelium of both groups than in stromal smooth cells (p<0.05).
CONCLUSIONS
Finasteride treatment had no clear effect on the expression of VEGF or 5AR type II. It is possible, however, that finasteride improves blood loss after TURP and BPH-induced hematuria by reducing MVD. Further study on the mechanism of MVD reduction is needed.
Keyword
MeSH Terms
-
3-Oxo-5-alpha-Steroid 4-Dehydrogenase
Blood Vessels
Epithelium
Finasteride
Hematuria
Hemorrhage
Humans
Microvessels
Paraffin
Prostatic Hyperplasia
Transurethral Resection of Prostate
Vascular Endothelial Growth Factor A
3-Oxo-5-alpha-Steroid 4-Dehydrogenase
Finasteride
Paraffin
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Madsen FA, Bruskewitz RC. Benign prostatic hyperplasia: pathophysiology and pharmacological treatment. Curr Opin Nephrol Hypertens. 1995. 4:455–459.2. Foley SJ, Soloman LZ, Wedderburn AW, Kashif KM, Summerton D, Basketter V, et al. A prospective study of the natural history of hematuria associated with benign prostatic hyperplasia and the effect of finasteride. J Urol. 2000. 163:496–498.3. Marshall S, Narayan P. Treatment of prostatic bleeding: suppression of angiogenesis by androgen deprivation. J Urol. 1993. 149:1553–1554.4. Puchner PJ, Miller MI. The effects of finasteride on hematuria associated with benign prostatic hyperplasia: a preliminary report. J Urol. 1995. 154:1779–1782.5. Cha WH, Jang TJ, Lee KS. Expression of survivin and Bcl-2 in benign prostatic hyperplasia treated with a 5-alpha-reductase inhibitor. Korean J Urol. 2008. 49:242–247.6. Huynh H, Seyam RM, Brock GB. Reduction of ventral prostate weight by finasteride is associated with suppression of insulin-like growth factor 1 (IGF-1) and IGF-1 receptor genes and with an increase in IGF binding protein 3. Cancer Res. 1998. 58:215–218.7. Schäfer W, Tammela TL, Barrett DM, Abrams P, Hedlund H, Rollema HJ, et al. Finasteride Urodynamics Study Group. Continued improvement in pressure-flow parameters in men receiving finasteride for 2 years. Urology. 1999. 54:278–283.8. Sandhu JS, Te AE. The role of 5-alpha-reductase inhibition as monotherapy in view of the MTOPS data. Curr Urol Rep. 2004. 5:274–279.9. Miller MI, Puchner PJ. Effects of finasteride on hematuria associated with benign prostatic hyperplasia: long-term follow-up. Urology. 1998. 51:237–240.10. Hochberg DA, Basillote JB, Armenakas NA, Vasovic L, Shevchuk M, Pareek G, et al. Decreased suburethral prostatic microvessel density in finasteride treated prostates: a possible mechanism for reduced bleeding in benign prostatic hyperplasia. J Urol. 2002. 167:1731–1733.11. Pareek G, Shevchuk M, Armenakas NA, Vasjovic L, Hochberg DA, Basillote JB, et al. The effect of finasteride on the expression of vascular endothelial growth factor and microvessel density: a possible mechanism for decreased prostatic bleeding in treated patients. J Urol. 2003. 169:20–23.12. Canda AE, Mungan MU, Yilmaz O, Yorukoglu K, Tuzel E, Kirkali Z. Effects of finasteride on the vascular surface density, number of microvessels and vascular endothelial growth factor expression of the rat prostate. Int Urol Nephrol. 2006. 38:275–280.13. Vaarala MH, Lukkarinen O, Marttila T, Kyllönen AP, Porvari KS, Vihko PT. Prostatic expression of human 5alpha-reductase type 2 during finasteride therapy: a randomized, double-blind, placebo-controlled study. World J Urol. 2000. 18:406–410.14. Bigler SA, Deering RE, Brawer MK. Comparison of microscopic vascularity in benign and malignent prostate tissue. Hum Pathol. 1993. 24:220–226.15. Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989. 141:243–247.16. Carlin BI, Bodner DR, Spirnak JP, Resnick MI. Role of finasteride in the treatment of recurrent hematuria secondary to benign prostatic hyperplasia. Prostate. 1997. 31:180–182.17. Kearney MC, Bingham JB, Bergland R, Meade-D'Alisera P, Puchner PJ. Clinical predictors in the use of finasteride for control of gross hematuria due to benign prostatic hyperplasia. J Urol. 2002. 167:2489–2491.18. Gormley GJ, Stoner E, Bruskewitz RC, Imperato-McGinley J, Walsh PC, McConnell JD, et al. The Finasteride Study Group. The effect of finasteride in men with benign prostatic hyperplasia. N Engl J Med. 1992. 327:1185–1191.19. Sandfeldt L, Bailey DM, Hahn RG. Blood loss during transurethral resection of the prostate after 3 months of treatment with finasteride. Urology. 2001. 58:972–976.20. Hagerty JA, Ginsberg PC, Harmon JD, Harkaway RC. Pretreatment with finasteride decreases perioperative bleeding associated with transurethral resection of the prostate. Urology. 2000. 55:684–689.21. Donohue JF, Hayne D, Karnik U, Thomas DR, Foster MC. Randomized, placebo-controlled trial showing that finasteride reduces prostatic vascularity rapidly within 2 weeks. BJU Int. 2005. 96:1319–1322.22. Woo JH, Kang JY, Kim EK, Yoo TK. The effect of short term dutasteride therapy on microvessel density in benign prostatic hyperplasia. Korean J Urol. 2008. 49:515–519.23. Weisser H, Krieg M. Kinetic analysis of androstenedione 5 alpha-reductase in epithelium and stroma of human prostate. Steroids. 1997. 62:589–594.24. Ferrara N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999. 56:794–814.25. Ravindranath N, Wion D, Brachet P, Djakiew D. Epidermal growth factor modulates the expression of vascular endothelial growth factor in the human prostate. J Androl. 2001. 22:432–443.26. Folkman J. Angiogenesis and breast cancer. J Clin Oncol. 1994. 12:441–443.27. Lekas AG, Lazaris AC, Chrisofos M, Papatsoris AG, Lappas D, Patsouris E, et al. Finasteride effects on hypoxia and angiogenetic markers in benign prostatic hyperplasia. Urology. 2006. 68:436–441.28. Weisser H, Krieg M. In vitro inhibition of androstenedione 5alpha-reduction by finasteride in epithelium and stroma of human benign prostatic hyperplasia. J Steroid Biochem Mol Biol. 1998. 67:49–55.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 5alpha-Reductase
- Expression of Vascular Endothelial Growth Factor (VEGF) and Microvessel Density in Hepatocellular Carcinoma
- Effect of Finasteride on Free/Total Serum PSA Ratio in Patients with Benign Prostate Hyperplasia
- Effect of 5alpha-Reductase Inhibitor in Expression of Transforming Growth Factor-beta1 in Benign Prostatic Hyperplasia Patients
- The role of tumor-associated macrophages on microvessel density after neoadjuvant chemotherapy in tongue cancer