Korean J Urol.
2006 Nov;47(11):1178-1184. 10.4111/kju.2006.47.11.1178.
Effect of 5alpha-Reductase Inhibitor in Expression of Transforming Growth Factor-beta1 in Benign Prostatic Hyperplasia Patients
- Affiliations
-
- 1Department of Urology, Myeonggok Clinical Institute, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2294276
- DOI: http://doi.org/10.4111/kju.2006.47.11.1178
Abstract
- Purpose
Transforming growth factor (TGF)-beta is a member of the superfamily of polypeptides, which control cell cycle progression and a variety of other cellular activities. TGF-beta1 has been implicated as an effector of the induction of apoptosis in response to 5alpha-reductase inhibitor (5ARI) and; therefore, causes a decrease in the prostate volume. We investigated the effect of 5ARI in the expression of TGF-beta1 in benign prostatic hyperplasia (BPH).
Materials and Methods
50 patients diagnosed with BPH were divided into two groups. The control group (n=30), in which a transurethral resection of the prostate (TURP) was performed without medication, and the 5ARI group (n=20), who were administrated with 5 mg of 5ARI daily for at least 3 months, followed by TURP. The resected specimens were stained with anti-rabbit TGF-beta1 polyclonal antibody using immunofluoroscent staining. The expression of TGF-beta1 was analyzed with a confocal laser scanning microscope and an image analyzer. The mRNA level of TGF-beta1 was determined by reverse transcriptase-polymerase chain reaction (RT-PCR).
Results
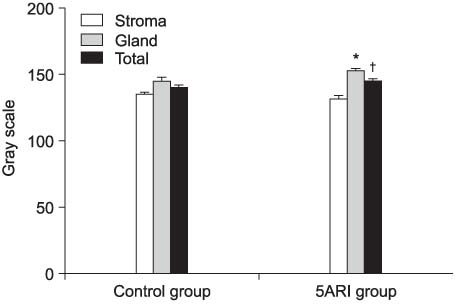
There were no statistical differences in the patient characteristics, including age, serum prostate-specific antigen (PSA) level and prostate volume, between the two groups. The expression of TGF-beta1 was demonstrated in the luminal epithelium and smooth muscle cells in BPH. TGF-beta1 was more strongly expressed in the luminal epithelium of both groups, and in the 5ARI group than the control (p<0.001).
Conclusions
These results suggest that 5ARI up-regulates the expression of TGF-beta1 in BPH patients, and may a play role as an inhibitor in the proliferation of BPH through the TGF-beta1 signal pathway.
MeSH Terms
-
Apoptosis
Cell Cycle
Epithelium
Humans
Myocytes, Smooth Muscle
Peptides
Phenobarbital
Prostate
Prostate-Specific Antigen
Prostatic Hyperplasia*
RNA, Messenger
Signal Transduction
Transforming Growth Factor beta1
Transforming Growth Factors
Transurethral Resection of Prostate
Peptides
Phenobarbital
Prostate-Specific Antigen
RNA, Messenger
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. Lee KL, Peehl DM. Molecular and cellular pathogenesis of benign prostatic hyperplasia. J Urol. 2004. 172:1784–1791.2. Chapple CR. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: an overview for the practising clinician. BJU Int. 2004. 94:738–744.3. Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001. 58:6 Suppl 1. 17–24.4. Djonov V, Ball RK, Graf S, Mottaz AE, Arnold AM, Flanders K, et al. Transforming growth factor-beta 3 is expressed in nondividing basal epithelial cells in normal human prostate and benign prostatic hyperplasia, and is no longer detectable in prostate carcinoma. Prostate. 1997. 31:103–109.5. Hong SJ. Benign prostatic hyperplasia: multiple factors for prostate tissue change with aging. Korean J Urol. 2005. 46:547–554.6. Reynolds AR, Kyprianou N. Growth factor signalling in prostatic growth: significance in tumour development and therapeutic targeting. Br J Pharmacol. 2006. 147:Suppl 2. S144–S152.7. Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005. 40:121–128.8. Saez C, Gonzalez-Baena AC, Japon MA, Giraldez J, Segura DI, Miranda G, et al. Regressive changes in finasteride-treated human hyperplastic prostates correlate with an upregulation of TGF-b receptor expression. Prostate. 1998. 37:84–90.9. Lucia MS, Sporn MB, Roberts AB, Stewart LV, Danielpour D. The role of transforming growth factor-beta1, -beta2, and -beta3 in androgen-responsive growth of NRP-152 rat prostatic epithelial cells. J Cell Physiol. 1998. 175:184–192.10. Speakman MJ, Kirby RS, Joyce A, Abrams P, Pocock R. Guideline for the primary care management of male lower urinary tract symptoms. BJU Int. 2004. 93:985–990.11. Alan WP, Ronald R. Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. The molecular biology, endocrinology, and physiology of the prostate and seminal vesicles. Campbell's urology. 2002. 8th ed. Philadelphia: Saunders;1237–1296.12. Danielpour D. Functions and regulation of transforming growth factor-beta (TGF-b) in the prostate. Eur J Cancer. 2005. 41:846–857.13. Sporn MB, Roberts AB. Interactions of retinoids and transforming growth factor-β in regulation of cell differentiation and proliferation. Mol Endocrinol. 1991. 5:3–7.14. Sporn MB, Roberts AB. TGF-β: problems and prospects. Cell Regul. 1990. 1:875–882.15. Zhou W, Park I, Pins M, Kozlowski JM, Jovanovic B, Zhang J, et al. Dual regulation of proliferation and growth arrest in prostatic stromal cells by transforming growth factor-beta1. Endocrinology. 2003. 144:4280–4284.16. Itoh N, Patel U, Cupp AS, Skinner MK. Developmental and hormonal regulation of transforming growth factor-beta1 (TGF beta1), -2, and -3 gene expression in isolated prostatic epithelial and stromal cells: epidermal growth factor and TGF beta interactions. Endocrinology. 1998. 139:1378–1388.17. Story MT, Hopp KA, Molter M. Expression of transforming growth factor beta 1 (TGF-β1), -β2, and -β3 by cultured human prostate cells. J Cell Physiol. 1996. 169:97–107.18. Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, et al. Mesenchymal-epithelial interactions and transforming growth factor-beta expression during mouse prostate morphogenesis. Endocrinology. 1994. 134:1039–1045.19. Kyprianou N, Tu H, Jacobs SC. Apoptotic versus proliferative activities in human benign prostatic hyperplasia. Hum Pathol. 1996. 27:668–675.20. McNeal J. Pathology of benign prostatic hyperplasia. Insight into etiology. Urol Clin North Am. 1990. 17:477–486.21. Price H, McNeal JE, Stamey TA. Evolving patterns of tissue composition in benign prostatic hyperplasia as a function of specimen size. Hum Pathol. 1990. 21:578–585.22. Kaplan SA. 5a-reductase inhibitor: What role should they play? Urology. 2001. 58:6 Suppl 1. 65–70.23. Lowe FC, McConnell JD, Hudson PB, Romas NA, Boake R, Lieber M, et al. Long-term 6-year experience with finasteride in patients with benign prostatic hyperplasia. Urology. 2003. 61:791–796.24. Boyle P, Roehrborn C, Harkaway R, Logie J, de la Rosette J, Emberton M. 5-Alpha reductase inhibition provides superior benefits to alpha blockade by preventing AUR and BPH-related surgery. Eur Urol. 2004. 45:620–627.25. Miller MI, Puchner PJ. Effects of finasteride on hematuria associated with benign prostatic hyperplasia: long-term follow-up. Urology. 1998. 51:237–240.26. Sandfeldt L, Bailey DM, Hahn RG. Blood loss during transurethral resection of the prostate after 3 months of treatment with finasteride. Urology. 2001. 58:972–976.27. Zhu B, Kyprianou N. Transforming growth factor beta and prostate cancer. Cancer Treat Res. 2005. 126:157–173.28. Kaminska B, Wesolowska A, Danilkiewicz M. TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol. 2005. 52:329–337.29. Glassman DT, Chon JK, Borkowski A, Jacobs SC, Kyprianou N. Combined effect of terazosin and finasteride on apoptosis, cell proliferation, and transforming growth factor-beta expression in benign prostatic hyperplasia. Prostate. 2001. 46:45–51.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 5alpha-Reductase
- The Effect of Finasteride on Microvessel Density and Expression of Vascular Endothelial Growth Factor and 5alpha-Reductase in Prostatic Hyperplasia
- Expression of Transforming Growth Factor -beta1, -beta2 in Human Endometrium of The Uterine Adenocarcinoma
- Inhibition of Cell Growth and Suppression of c-myc Gene Expression by Transforming Growth Factor-beta1 in Cervical Carcinoma Cell Lines
- Changes of Urinary Nerve Growth Factor and Transforming Growth Factor beta-1 in Male Patients with Lower Urinary Tract Symptom