Nutr Res Pract.
2015 Oct;9(5):480-488. 10.4162/nrp.2015.9.5.480.
Protective role of caffeic acid in an Abeta25-35-induced Alzheimer's disease model
- Affiliations
-
- 1Department of Food Science and Nutrition, and Kimchi Research Institute, Pusan National University, Busandaehak-ro 63 beon-gil, Geumjeong-gu, Busan 609-735, Korea. ejcho@pusan.ac.kr
- 2Department of Integrative Plant Science, Chung-Ang University, Seodong-daero 4726, Daedeok-myeon, Anseong 456-756, Korea.
- KMID: 2313868
- DOI: http://doi.org/10.4162/nrp.2015.9.5.480
Abstract
- BACKGROUND/OBJECTIVES
Alzheimer's disease (AD) is characterized by deficits in memory and cognitive functions. The accumulation of amyloid beta peptide (Abeta) and oxidative stress in the brain are the most common causes of AD.
MATERIALS/METHODS
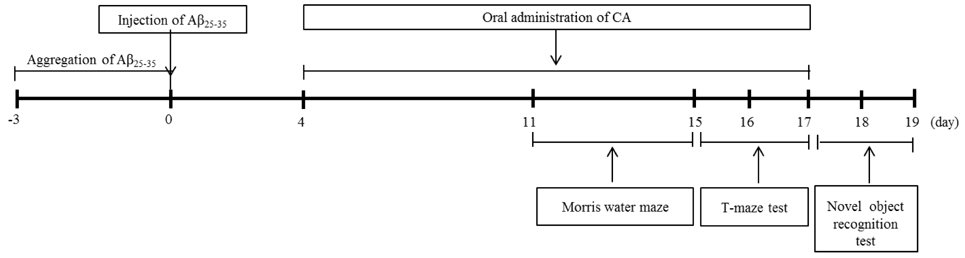
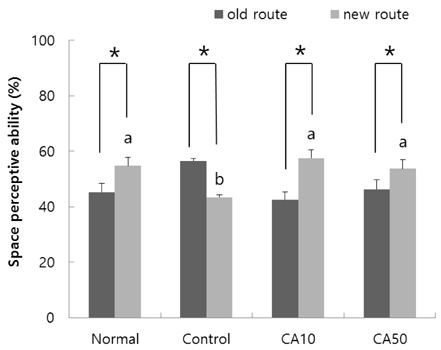
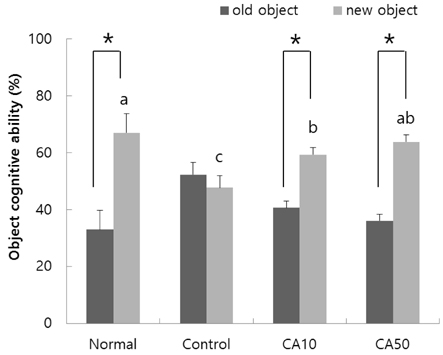
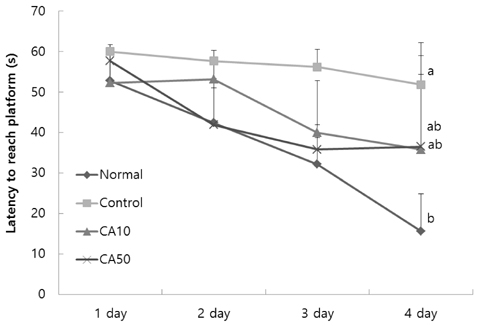
Caffeic acid (CA) is an active phenolic compound that has a variety of pharmacological actions. We studied the protective abilities of CA in an Abeta25-35-injected AD mouse model. CA was administered at an oral dose of 10 or 50 mg/kg/day for 2 weeks. Behavioral tests including T-maze, object recognition, and Morris water maze were carried out to assess cognitive abilities. In addition, lipid peroxidation and nitric oxide (NO) production in the brain were measured to investigate the protective effect of CA in oxidative stress.
RESULTS
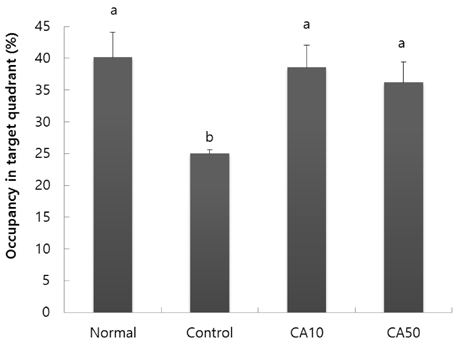
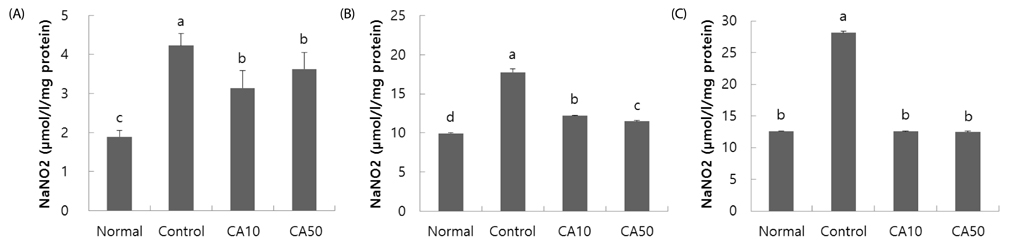
In the T-maze and object recognition tests, novel route awareness and novel object recognition were improved by oral administration of CA compared with the Abeta25-35-injected control group. These results indicate that administration of CA improved spatial cognitive and memory functions. The Morris water maze test showed that memory function was enhanced by administration of CA. In addition, CA inhibited lipid peroxidation and NO formation in the liver, kidney, and brain compared with the Abeta25-35-injected control group. In particular, CA 50 mg/kg/day showed the stronger protective effect from cognitive impairment than CA 10 mg/kg/day.
CONCLUSIONS
The present results suggest that CA improves Abeta25-35-induced memory deficits and cognitive impairment through inhibition of lipid peroxidation and NO production.
MeSH Terms
Figure
Reference
-
1. Tsunekawa H, Noda Y, Mouri A, Yoneda F, Nabeshima T. Synergistic effects of selegiline and donepezil on cognitive impairment induced by amyloid beta (25-35). Behav Brain Res. 2008; 190:224–232.
Article2. Doraiswamy PM. Non-cholinergic strategies for treating and preventing Alzheimer's disease. CNS Drugs. 2002; 16:811–824.
Article3. Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994; 53:438–447.
Article4. Takahata K, Minami A, Kusumoto H, Shimazu S, Yoneda F. Effects of selegiline alone or with donepezil on memory impairment in rats. Eur J Pharmacol. 2005; 518:140–144.
Article5. Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001; 30:447–450.6. Nunomura A, Castellani RJ, Zhu X, Moreira PI, Perry G, Smith MA. Involvement of oxidative stress in Alzheimer disease. J Neuropathol Exp Neurol. 2006; 65:631–641.
Article7. Avdulov NA, Chochina SV, Igbavboa U, O'Hare EO, Schroeder F, Cleary JP, Wood WG. Amyloid beta-peptides increase annular and bulk fluidity and induce lipid peroxidation in brain synaptic plasma membranes. J Neurochem. 1997; 68:2086–2091.
Article8. Butterfield DA. Beta-amyloid-associated free radical oxidative stress and neurotoxicity: implications for Alzheimer's disease. Chem Res Toxicol. 1997; 10:495–506.
Article9. Butterfield DA. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity" implications for neurodegeneration in Alzheimer's disease brain. A review. Free Radic Res. 2002; 36:1307–1313.10. Markesbery WR, Carney JM. Oxidative alterations in Alzheimer's disease. Brain Pathol. 1999; 9:133–146.
Article11. Behl C, Davis J, Cole GM, Schubert D. Vitamin E protects nerve cells from amyloid beta protein toxicity. Biochem Biophys Res Commun. 1992; 186:944–950.12. Kim J, Lee HJ, Lee KW. Naturally occurring phytochemicals for the prevention of Alzheimer's disease. J Neurochem. 2010; 112:1415–1430.
Article13. Butterfield D, Castegna A, Pocernich C, Drake J, Scapagnini G, Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer's disease. J Nutr Biochem. 2002; 13:444–461.
Article14. Moridani MY, Scobie H, Jamshidzadeh A, Salehi P, O'Brien PJ. Caffeic acid, chlorogenic acid, and dihydrocaffeic acid metabolism: glutathione conjugate formation. Drug Metab Dispos. 2001; 29:1432–1439.15. Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005; 45:287–306.
Article16. Touaibia M, Jean-François J, Doiron J. Caffeic acid, a versatile pharmacophore: an overview. Mini Rev Med Chem. 2011; 11:695–713.
Article17. U Rehman M, Sultana S. Attenuation of oxidative stress, inflammation and early markers of tumor promotion by caffeic acid in Fe-NTA exposed kidneys of Wistar rats. Mol Cell Biochem. 2011; 357:115–124.
Article18. Roos TU, Heiss EH, Schwaiberger AV, Schachner D, Sroka IM, Oberan T, Vollmar AM, Dirsch VM. Caffeic acid phenethyl ester inhibits PDGF-induced proliferation of vascular smooth muscle cells via activation of p38 MAPK, HIF-1α, and heme oxygenase-1. J Nat Prod. 2011; 74:352–356.
Article19. Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011; 44:192–201.
Article20. Eom TK, Ryu B, Lee JK, Byun HG, Park SJ, Kim SK. β-secretase inhibitory activity of phenolic acid conjugated chitooligosaccharides. J Enzyme Inhib Med Chem. 2013; 28:214–217.
Article21. Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009; 84:257–262.
Article22. Khan KA, Kumar N, Nayak PG, Nampoothiri M, Shenoy RR, Krishnadas N, Rao CM, Mudgal J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J Pharm Pharmacol. 2013; 65:1745–1752.
Article23. Muthaiyah B, Essa MM, Lee M, Chauhan V, Kaur K, Chauhan A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer's disease. J Alzheimers Dis. 2014; 42:1397–1405.
Article24. Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered β-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996; 706:181–193.
Article25. Laursen SE, Belknap JK. Intracerebroventricular injections in mice. Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357.26. Montgomery KC. A test of two explanations of spontaneous alternation. J Comp Physiol Psychol. 1952; 45:287–293.
Article27. Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat Protoc. 2006; 1:1306–1311.
Article28. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.
Article29. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article30. Schmidt HH, Warner TD, Nakane M, Förstermann U, Murad F. Regulation and subcellular location of nitrogen oxide synthases in RAW264.7 macrophages. Mol Pharmacol. 1992; 41:615–624.31. Bachman DL, Wolf PA, Linn RT, Knoefel JE, Cobb JL, Belanger AJ, White LR, D'Agostino RB. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993; 43:515–519.
Article32. Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, Zou LB, Nabeshima T. Xanthoceraside attenuates amyloid β peptide(25-35)-induced learning and memory impairments in mice. Psychopharmacology (Berl). 2012; 219:181–190.
Article33. Kubo T, Nishimura S, Kumagae Y, Kaneko I. In vivo conversion of racemized beta-amyloid ([D-Ser 26]A beta 1-40) to truncated and toxic fragments ([D-Ser 26]A beta 25-35/40) and fragment presence in the brains of Alzheimer's patients. J Neurosci Res. 2002; 70:474–483.
Article34. Mattson MP, Begley JG, Mark RJ, Furukawa K. Abeta25-35 induces rapid lysis of red blood cells: contrast with Aβ1-42 and examination of underlying mechanisms. Brain Res. 1997; 771:147–153.
Article35. Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004; 3:205–214.
Article36. Wypijewska A, Galazka-Friedman J, Bauminger ER, Wszolek ZK, Schweitzer KJ, Dickson DW, Jaklewicz A, Elbaum D, Friedman A. Iron and reactive oxygen species activity in parkinsonian substantia nigra. Parkinsonism Relat Disord. 2010; 16:329–333.
Article37. Choi SJ, Kim MJ, Heo HJ, Kim JK, Jun WJ, Kim HK, Kim EK, Kim MO, Cho HY, Hwang HJ, Kim YJ, Shin DH. Ameliorative effect of 1,2-benzenedicarboxylic acid dinonyl ester against amyloid beta peptide-induced neurotoxicity. Amyloid. 2009; 16:15–24.
Article38. Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995; 322:339–346.
Article39. Ono K, Hasegawa K, Naiki H, Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Biochim Biophys Acta. 2004; 1690:193–202.
Article40. Ono K, Yoshiike Y, Takashima A, Hasegawa K, Naiki H, Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer's disease. J Neurochem. 2003; 87:172–181.
Article41. Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, Kim SH, Kim JG, Kim CH. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004; 18:1670–1681.
Article42. Huang Y, Jin M, Pi R, Zhang J, Chen M, Ouyang Y, Liu A, Chao X, Liu P, Liu J, Ramassamy C, Qin J. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci Lett. 2013; 535:146–151.
Article43. Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002; 26:91–104.
Article44. Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009; 5:18–29.
Article45. Selkoe DJ, Podlisny MB, Joachim CL, Vickers EA, Lee G, Fritz LC, Oltersdorf T. Beta-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A. 1988; 85:7341–7345.
Article46. Sandbrink R, Masters CL, Beyreuther K. Beta A4-amyloid protein precursor mRNA isoforms without exon 15 are ubiquitously expressed in rat tissues including brain, but not in neurons. J Biol Chem. 1994; 269:1510–1517.
Article47. Butterfield DA, Boyd-Kimball D. Amyloid beta-peptide(1-42) contributes to the oxidative stress and neurodegeneration found in Alzheimer disease brain. Brain Pathol. 2004; 14:426–432.
Article48. Cotman CW, Su JH. Mechanisms of neuronal death in Alzheimer's disease. Brain Pathol. 1996; 6:493–506.
Article49. Choi JY, Cho EJ, Lee HS, Lee JM, Yoon YH, Lee S. Tartary buckwheat improves cognition and memory function in an in vivo amyloid-β-induced Alzheimer model. Food Chem Toxicol. 2013; 53:105–111.
Article50. Lee AY, Yamabe N, Kang KS, Kim HY, Lee S, Cho EJ. Cognition and memory function of Taraxacum coreanum in an in vivo amyloid-β-induced mouse model of Alzheimer's disease. Arch Biol Sci. 2014; 66:1357–1366.
Article51. Choi YY, Maeda T, Fujii H, Yokozawa T, Kim HY, Cho EJ, Shibamoto T. Oligonol improves memory and cognition under an amyloid β(25-35)-induced Alzheimer's mouse model. Nutr Res. 2014; 34:595–603.
Article52. Soliman KF, Mazzio EA. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc Soc Exp Biol Med. 1998; 218:390–397.
Article53. Yan JJ, Cho JY, Kim HS, Kim KL, Jung JS, Huh SO, Suh HW, Kim YH, Song DK. Protection against β-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br J Pharmacol. 2001; 133:89–96.
Article54. Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004; 79:727–747.
Article55. National Institute of Food and Drug Safety Evaluation (KR). Tox-Info. Caffeic acid. [internet]. Cheongju: National Institute of Food and Drug Safety Evaluation;2015. cited 2015 March 22. Available from: http://www.nifds.go.kr/toxinfo/index.56. Shinomiya K, Omichi J, Ohnishi R, Ito H, Yoshida T, Kamei C. Effects of chlorogenic acid and its metabolites on the sleep-wakefulness cycle in rats. Eur J Pharmacol. 2004; 504:185–189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Flavonoid Compounds on beta-amyloid-peptide-induced Neuronal Death in Cultured Mouse Cortical Neurons
- Protective Effect of Ginsenoside Rb1 and Rg1 Against beta Amyloid ( 25-35 )-Induced Neurotoxicity on B103 cells
- Gene Expression Analysis of the Human Astrocytoma Cell after Abeta25-35 Stimulation Followed by Ibuprofen Administration
- Protective Role of Microglia on Neuronal Survival after Exposure to Amyloid Beta
- Protective Effect of Fibroin BF-7 on Neuronal Cell Death in Alzheimer Model using Amyloid beta Peptide