Nutr Res Pract.
2014 Oct;8(5):550-557. 10.4162/nrp.2014.8.5.550.
Bioavailability of plant pigment phytochemicals in Angelica keiskei in older adults: A pilot absorption kinetic study
- Affiliations
-
- 1Jean Mayer USDA- Human Nutrition Research Center on Aging, Tufts University, 711 Washington St., Boston, MA 02111, USA.
- 2Department of Internal Medicine, Botucatu Medical School, Sao Paulo State University (UNESP), Distrito Rubiao Jr. s/n, 18618-970 Botucatu, SP, Brazil.
- 3Department of Pharmaceutical Sciences, Universita degli Studi di Milano, 20133 Milan, Italy.
- 4National Academy of Agricultural Science, Rural Development Administration, Suwon 441-853, Republic of Korea.
- 5College of Biomedical and Health Sciences, Konkuk University, Chungju-si, 380-701, Republic of Korea. kyeum@kku.ac.kr
- KMID: 2313778
- DOI: http://doi.org/10.4162/nrp.2014.8.5.550
Abstract
- BACKGROUND/OBJECTIVES
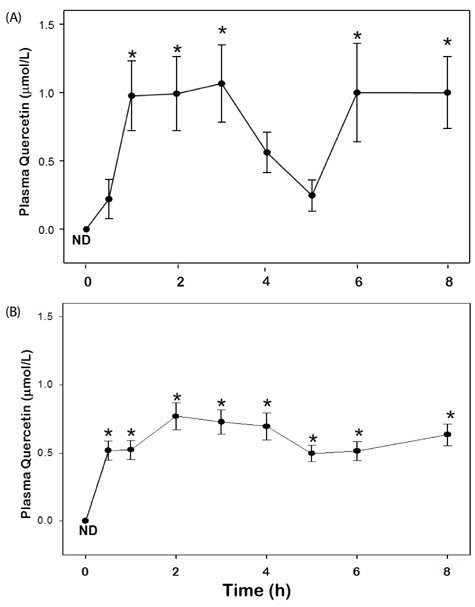
Angelica keiskei is a green leafy vegetable rich in plant pigment phytochemicals such as flavonoids and carotenoids. This study examined bioavailability of flavonoids and carotenoids in Angelica keiskei and the alteration of the antioxidant performance in vivo. SUBJECTS AND MATERIALS: Absorption kinetics of phytochemicals in Angelica keiskei were determined in healthy older adults (> 60 y, n = 5) and subjects with metabolic syndrome (n = 5). Subjects consumed 5 g dry Angelica keiskei powder encapsulated in gelatin capsules with a low flavonoid and carotenoid liquid meal. Plasma samples were collected at baseline, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h. Samples were analyzed for flavonoids and carotenoids using HPLC systems with electrochemical and UV detection, respectively, and for total antioxidant performance by fluorometry.
RESULTS
After ingestion of Angelica keiskei increases in plasma quercetin concentrations were observed at 1-3 and 6-8 hr in the healthy group and at all time points in the metabolic syndrome group compared to baseline (P < 0.05). Plasma lutein concentrations were significantly elevated in both the healthy and metabolic syndrome groups at 8 hr (P < 0.05). Significant increases in total antioxidant performance were also observed in both the healthy and the metabolic syndrome groups compared to baseline (P < 0.05).
CONCLUSIONS
Findings of this study clearly demonstrate the bioavailability of phytonutrients of Angelica keiskei and their ability to increase antioxidant status in humans.
MeSH Terms
Figure
Reference
-
1. Hata K, Kozawa M. Pharmacognostical studies on umbelliferous plants. XVIII. On the constituents of the roots of Angelica Keiskei Koidzumi. Yakugaku Zasshi. 1961; 81:1647–1649.
Article2. Kozawa M, Morita N, Baba K, Hata K. Chemical components of the roots of Angelica keiskei Koidzumi. II. The structure of the chalcone derivatives (author's transl). Yakugaku Zasshi. 1978; 98:210–214.
Article3. Kozawa M, Morita N, Baba K, Hata K. Chemical components of the roots of Angelica keiskei Koidzumi. III. The structure of a new dihydrofurocoumarin (author's transl). Yakugaku Zasshi. 1978; 98:636–638.
Article4. Motani K, Tabata K, Kimura Y, Okano S, Shibata Y, Abiko Y, Nagai H, Akihisa T, Suzuki T. Proteomic analysis of apoptosis induced by xanthoangelol, a major constituent of Angelica keiskei, in neuroblastoma. Biol Pharm Bull. 2008; 31:618–626.
Article5. Kimura Y. New anticancer agents: in vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo. 2005; 19:37–60.6. Akihisa T, Tokuda H, Hasegawa D, Ukiya M, Kimura Y, Enjo F, Suzuki T, Nishino H. Chalcones and other compounds from the exudates of Angelica keiskei and their cancer chemopreventive effects. J Nat Prod. 2006; 69:38–42.
Article7. Fujita T, Sakuma S, Sumiya T, Nishida H, Fujimoto Y, Baba K, Kozawa M. The effects of xanthoangelol E on arachidonic acid metabolism in the gastric antral mucosa and platelet of the rabbit. Res Commun Chem Pathol Pharmacol. 1992; 77:227–240.8. Inamori Y, Baba K, Tsujibo H, Taniguchi M, Nakata K, Kozawa M. Antibacterial activity of two chalcones, xanthoangelol and 4-hydroxyderricin, isolated from the root of Angelica keiskei KOIDZUMI. Chem Pharm Bull (Tokyo). 1991; 39:1604–1605.
Article9. Kawabata K, Sawada K, Ikeda K, Fukuda I, Kawasaki K, Yamamoto N, Ashida H. Prenylated chalcones 4-hydroxyderricin and xanthoangelol stimulate glucose uptake in skeletal muscle cells by inducing GLUT4 translocation. Mol Nutr Food Res. 2011; 55:467–475.
Article10. Shimizu E, Hayashi A, Takahashi R, Aoyagi Y, Murakami T, Kimoto K. Effects of angiotensin I-converting enzyme inhibitor from Ashitaba (Angelica keiskei) on blood pressure of spontaneously hypertensive rats. J Nutr Sci Vitaminol (Tokyo). 1999; 45:375–383.
Article11. Kang MH, Park YK, Kim HY, Kim TS. Green vegetable drink consumption protects peripheral lymphocytes DNA damage in Korean smokers. Biofactors. 2004; 22:245–247.
Article12. Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005; 81:243S–255S.
Article13. Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005; 81:230S–242S.
Article14. Aldini G, Regazzoni L, Pedretti A, Carini M, Cho SM, Park KM, Yeum KJ. An integrated high resolution mass spectrometric and informatics approach for the rapid identification of phenolics in plant extract. J Chromatogr A. 2011; 1218:2856–2864.
Article15. Li L, Aldini G, Carini M, Oliver Chen CY, Chun HK, Cho SM, Park KM, Correa CR, Russell RM, Blumberg JB, Yeum KJ. Characterisation, extraction efficiency, stability and antioxidant activity of phytonutrients in Angelica keiskei. Food Chem. 2009; 115:227–232.
Article16. Yeum KJ, Aldini G, Chung HY, Krinsky NI, Russell RM. The activities of antioxidant nutrients in human plasma depend on the localization of attacking radical species. J Nutr. 2003; 133:2688–2691.
Article17. Yeum KJ, Beretta G, Krinsky NI, Russell RM, Aldini G. Synergistic interactions of antioxidant nutrients in a biological model system. Nutrition. 2009; 25:839–846.
Article18. Aldini G, Yeum KJ, Russell RM, Krinsky NI. A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic Biol Med. 2001; 31:1043–1050.
Article19. Beretta G, Aldini G, Facino RM, Russell RM, Krinsky NI, Yeum KJ. Total antioxidant performance: a validated fluorescence assay for the measurement of plasma oxidizability. Anal Biochem. 2006; 354:290–298.
Article20. Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among U.S. adults. Diabetes Care. 2004; 27:2444–2449.
Article21. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003-2006. Natl Health Stat Report. 2009; 1–7.22. Ford ES, Schulze MB, Pischon T, Bergmann MM, Joost HG, Boeing H. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovasc Diabetol. 2008; 7:35.
Article23. Grundy SM. A constellation of complications: the metabolic syndrome. Clin Cornerstone. 2005; 7:36–45.24. Malone DC, Boudreau DM, Nichols GA, Raebel MA, Fishman PA, Feldstein AC, Ben-Joseph RH, Okamoto LJ, Boscoe AN, Magid DJ. Association of cardiometabolic risk factors and prevalent cardiovascular events. Metab Syndr Relat Disord. 2009; 7:585–593.
Article25. Hansel B, Giral P, Nobecourt E, Chantepie S, Bruckert E, Chapman MJ, Kontush A. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J Clin Endocrinol Metab. 2004; 89:4963–4971.
Article26. Ford ES, Mokdad AH, Giles WH, Brown DW. The metabolic syndrome and antioxidant concentrations: findings from the Third National Health and Nutrition Examination Survey. Diabetes. 2003; 52:2346–2352.27. Coyne T, Ibiebele TI, Baade PD, McClintock CS, Shaw JE. Metabolic syndrome and serum carotenoids: findings of a cross-sectional study in Queensland, Australia. Br J Nutr. 2009; 102:1668–1677.
Article28. Zhao X, Aldini G, Johnson EJ, Rasmussen H, Kraemer K, Woolf H, Musaeus N, Krinsky NI, Russell RM, Yeum KJ. Modification of lymphocyte DNA damage by carotenoid supplementation in postmenopausal women. Am J Clin Nutr. 2006; 83:163–169.
Article29. Behrens WA, Madère R. A highly sensitive high-performance liquid chromatography method for the estimation of ascorbic and dehydroascorbic acid in tissues, biological fluids, and foods. Anal Biochem. 1987; 165:102–107.
Article30. Fossati P, Prencipe L, Berti G. Use of 3,5-dichloro-2-hydroxybenzenesulfonic acid/4-aminophenazone chromogenic system in direct enzymic assay of uric acid in serum and urine. Clin Chem. 1980; 26:227–231.
Article31. Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. J Nutr. 2005; 135:1366–1373.
Article32. Cho YS, Yeum KJ, Chen CY, Beretta G, Tang G, Krinsky NI, Yoon S, Lee-Kim YC, Blumberg JB, Russell RM. Phytonutrients affecting hydrophilic and lipophilic antioxidant activities in fruits, vegetables and legumes. J Sci Food Agric. 2007; 87:1096–1107.
Article33. Zhang Z, Kou X, Fugal K, McLaughlin J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J Agric Food Chem. 2004; 52:688–691.
Article34. Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C.: The National Academies Press;2000.35. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–1645.36. Torres S, Salgado-Ceballos H, Torres JL, Orozco-Suarez S, Díaz-Ruíz A, Martínez A, Rivera-Cruz M, Ríos C, Lara A, Collado C, Guizar-Sahagún G. Early metabolic reactivation versus antioxidant therapy after a traumatic spinal cord injury in adult rats. Neuropathology. 2010; 30:36–43.
Article37. Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004; 27:362–366.
Article38. Graefe EU, Wittig J, Mueller S, Riethling AK, Uehleke B, Drewelow B, Pforte H, Jacobasch G, Derendorf H, Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J Clin Pharmacol. 2001; 41:492–499.
Article39. Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, Piskula MK. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 2008; 138:885–888.
Article40. Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010; 328:228–231.
Article41. Dey A, Williams RS, Pollock DM, Stepp DW, Newman JW, Hammock BD, Imig JD. Altered kidney CYP2C and cyclooxygenase-2 levels are associated with obesity-related albuminuria. Obes Res. 2004; 12:1278–1289.
Article42. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article43. van Het Hof KH, West CE, Weststrate JA, Hautvast JG. Dietary factors that affect the bioavailability of carotenoids. J Nutr. 2000; 130:503–506.
Article44. van den Berg H. Effect of lutein on beta-carotene absorption and cleavage. Int J Vitam Nutr Res. 1998; 68:360–365.45. Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995; 62:604–610.
Article46. Marisiddaiah R, Baskaran V. Bioefficacy of beta-carotene is improved in rats after solubilized as equimolar dose of beta-carotene and lutein in phospholipid-mixed micelles. Nutr Res. 2009; 29:588–595.
Article47. Talegawkar SA, Beretta G, Yeum KJ, Johnson EJ, Carithers TC, Taylor HA Jr, Russell RM, Tucker KL. Total antioxidant performance is associated with diet and serum antioxidants in participants of the diet and physical activity substudy of the Jackson Heart Study. J Nutr. 2009; 139:1964–1971.
Article48. Dragsted LO, Pedersen A, Hermetter A, Basu S, Hansen M, Haren GR, Kall M, Breinholt V, Castenmiller JJ, Stagsted J, Jakobsen J, Skibsted L, Rasmussen SE, Loft S, Sandström B. The 6-a-day study: effects of fruit and vegetables on markers of oxidative stress and antioxidative defense in healthy nonsmokers. Am J Clin Nutr. 2004; 79:1060–1072.
Article49. McKay DL, Chen CY, Yeum KJ, Matthan NR, Lichtenstein AH, Blumberg JB. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010; 9:21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Evaluation of Metabolizable Energy of Angelica Keiskei (Angelica utilis Makino) Products
- Anticancer Activity of Phytochemicals of the Papaya Plant Assessed: A Narrative Review
- Xanthoangelol and 4-Hydroxyderricin Are the Major Active Principles of the Inhibitory Activities against Monoamine Oxidases on Angelica keiskei K
- The effects of Angelica keiskei Koidz on the expression of antioxidant enzymes related to lipid profiles in rats fed a high fat diet
- Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice