Nutr Res Pract.
2014 Aug;8(4):377-385.
Anti-proliferative and angio-suppressive effect of Stoechospermum marginatum (C. Agardh) Kutzing extract using various experimental models
- Affiliations
-
- 1Biological Oceanography Division, National Institute of Oceanography, Dona Paula, Panaji, Goa 403004, India. rashmi.vinayak@gmail.com
- 2Department of Studies in Biotechnology, University of Mysore, Manasagangothri, Mysore 570006, India.
Abstract
- BACKGROUND/OBJECTIVES
Abundant consumption of seaweeds in the diet is epidemiologically linked to the reduction in risk of developing cancer. In larger cases, however, identification of particular seaweeds that are accountable for these effects is still lacking, hindering the recognition of competent dietary-based chemo preventive approaches. The aim of this research was to establish the antiproliferative potency and angiosuppressive mode of action of Stoechospermum marginatum seaweed methanolic extract using various experimental models.
MATERIALS/METHODS
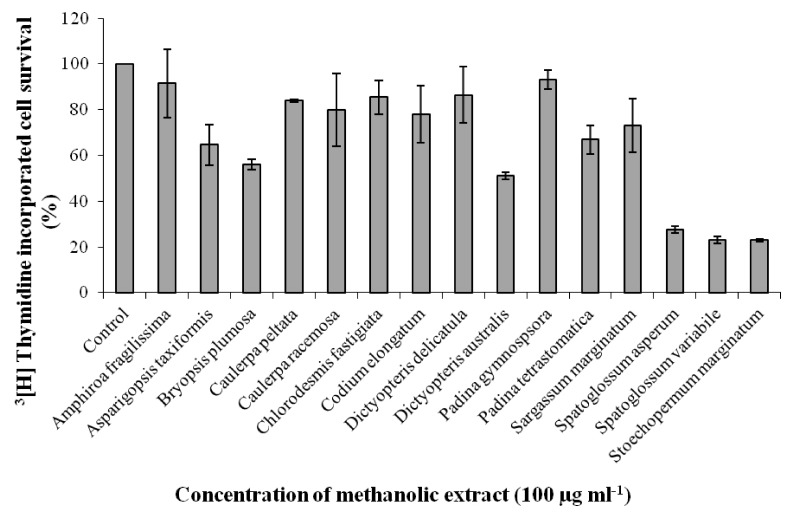
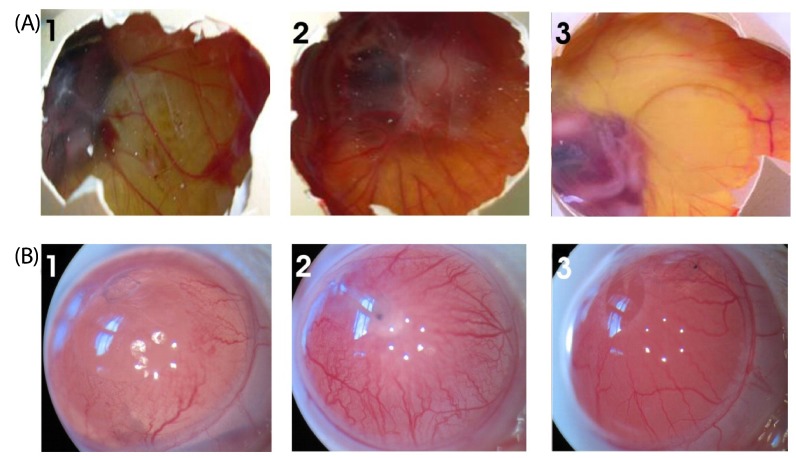
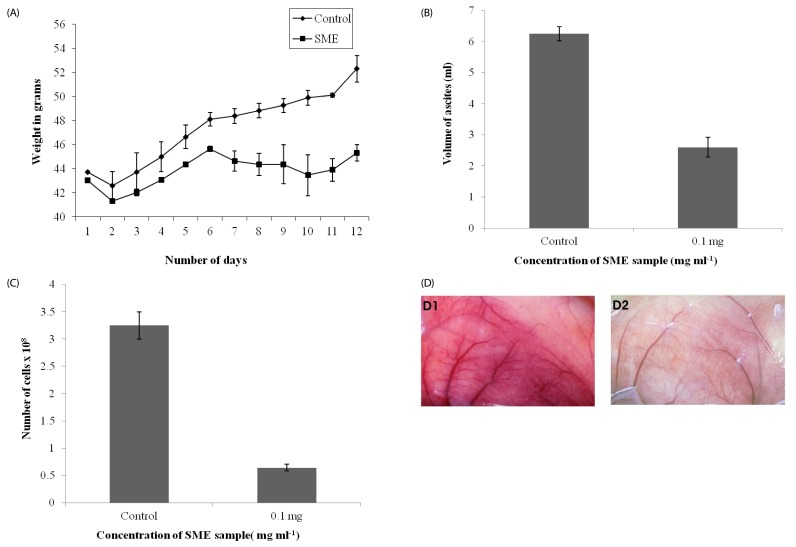
Among the 15 seaweeds screened for antiproliferative activity against Ehrlich ascites tumor (EAT) cell line, Stoechospermum marginatum extract (SME) was found to be the most promising. Therefore, it was further investigated for its anti-proliferative activity in-vitro against choriocarcinoma (BeWo) and non-transformed Human embryonic kidney (HEK 293) cells, and for its anti-migratory/tube formation activity against HUVEC cells in-vitro. Subsequently, the angiosuppressive activity of S. marginatum was established by inhibition of angiogenesis in in-vivo (peritoneal angiogenesis and chorioallantoic membrane assay) and ex-vivo (rat cornea assay) models.
RESULTS
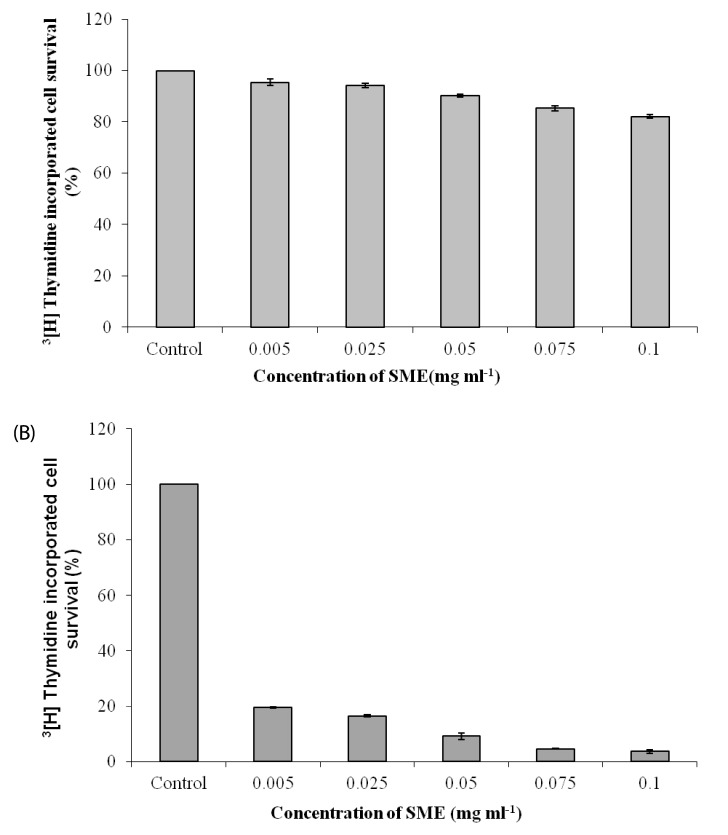
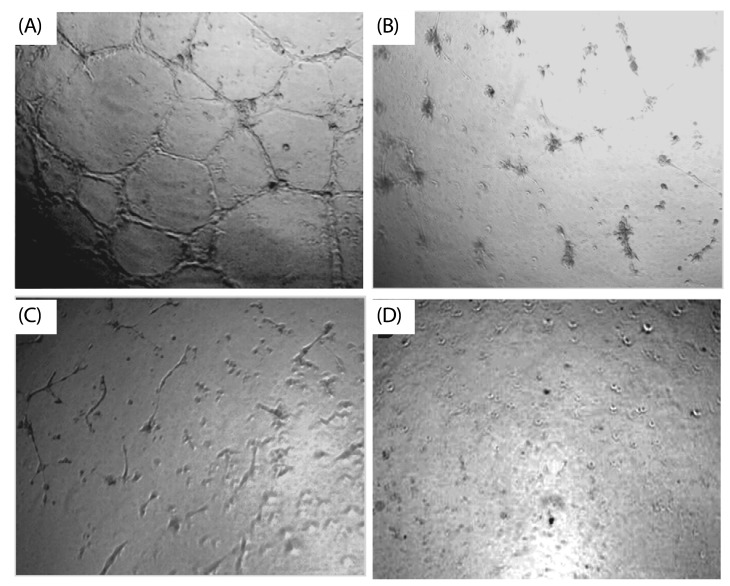
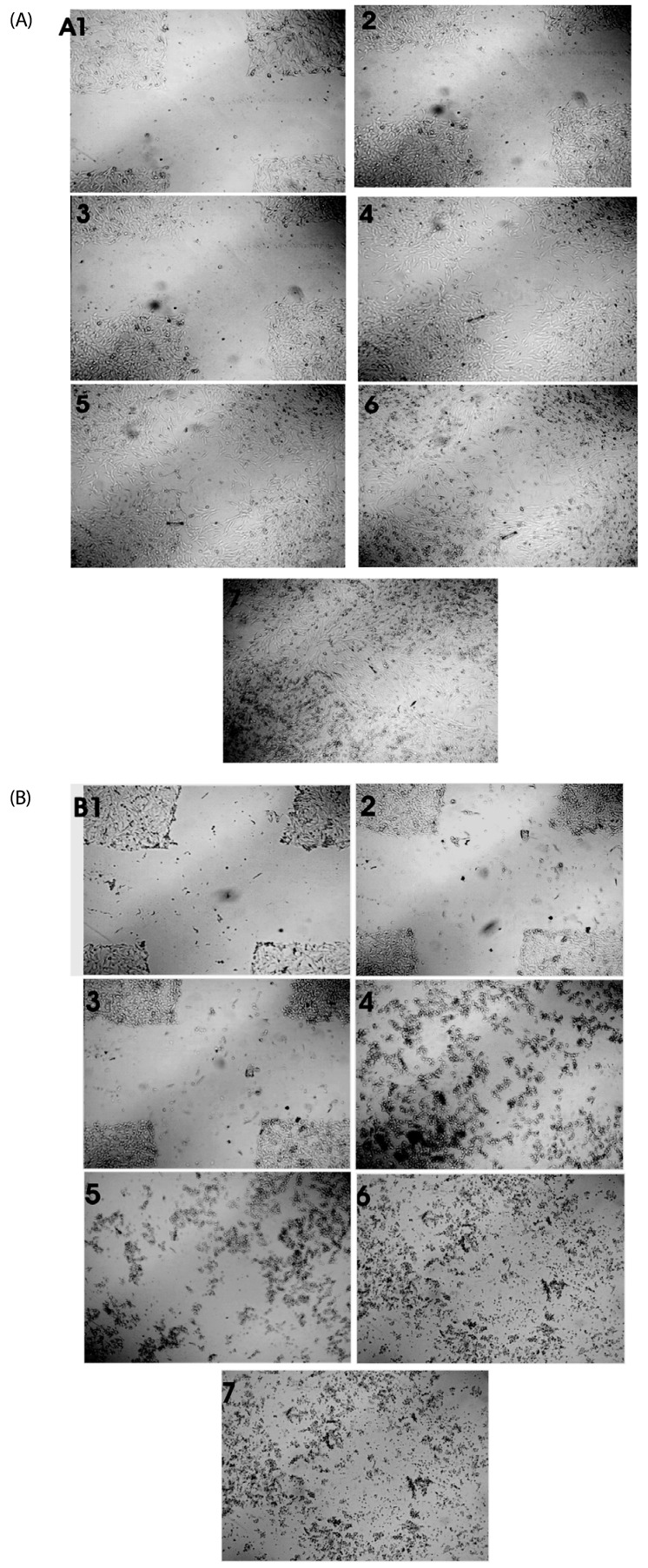
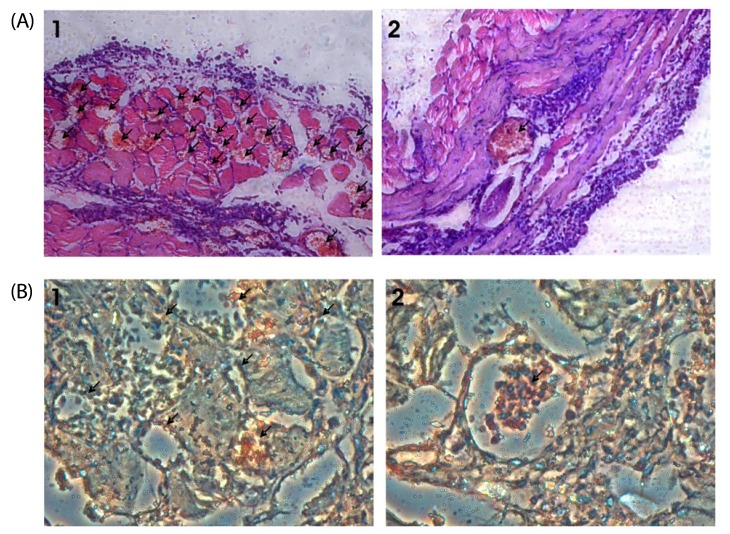
Most brown seaweed extracts inhibited the proliferation of EAT cells, while green and red seaweed extracts were much less effective. According to the results, SME selectively inhibited proliferation of BeWo cells in-vitro in a dose-dependent manner, but had a lesser effect on HEK 293 cells. SME also suppressed the migration and tube formation of HUVEC cells in-vitro. In addition, SME was able to suppress VEGF-induced angiogenesis in the chorio allantoic membrane, rat cornea, and tumor induced angiogenesis in the peritoneum of EAT bearing mice. A decrease in the microvessel density count and CD31 antigen staining of treated mice peritoneum provided further evidence of its angiosuppressive activity.
CONCLUSIONS
Altogether, the data underline that VEGF mediated angiogenesis is the target for the angiosuppressive action of SME and could potentially be useful in cancer prevention or treatment involving stimulated angiogenesis.
Keyword
MeSH Terms
-
Allantois
Animals
Antigens, CD31
Carcinoma, Ehrlich Tumor
Cell Line
Chorioallantoic Membrane
Choriocarcinoma
Cornea
Diet
Female
HEK293 Cells
Human Umbilical Vein Endothelial Cells
Humans
Kidney
Methanol
Mice
Microvessels
Models, Theoretical*
Peritoneum
Pregnancy
Rats
Seaweed
Vascular Endothelial Growth Factor A
Antigens, CD31
Methanol
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Nussenbaum F, Herman IM. Tumor angiogenesis: insights and innovations. J Oncol. 2010; 2010:132641. PMID: 20445741.
Article2. Gupta K, Zhang J. Angiogenesis: a curse or cure? Postgrad Med J. 2005; 81:236–242. PMID: 15811887.
Article3. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1:27–31. PMID: 7584949.
Article4. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996; 86:353–364. PMID: 8756718.
Article5. Folkman J, Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976; 16:207–248. PMID: 783062.6. Tanghetti E, Ria R, Dell'Era P, Urbinati C, Rusnati M, Ennas MG, Presta M. Biological activity of substrate-bound basic fibroblast growth factor (FGF2): recruitment of FGF receptor-1 in endothelial cell adhesion contacts. Oncogene. 2002; 21:3889–3897. PMID: 12032827.
Article7. Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000; 6:389–395. PMID: 10742145.
Article8. Keshet E, Ben-Sasson SA. Anticancer drug targets: approaching angiogenesis. J Clin Invest. 1999; 104:1497–1501. PMID: 10587512.
Article9. Madhusudan S, Harris AL. Drug inhibition of angiogenesis. Curr Opin Pharmacol. 2002; 2:403–414. PMID: 12127873.
Article10. Marwick C. Natural compounds show antiangiogenic activity. J Natl Cancer Inst. 2001; 93:1685. PMID: 11717328.
Article11. Smit AJ. Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol. 2004; 16:245–262.
Article12. Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008; 107:707–713.
Article13. Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008; 99:2717–2723. PMID: 17706415.
Article14. Kumar KS, Ganesan K, Subba Rao PV. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty - an edible seaweed. Food Chem. 2008; 107:289–295.
Article15. Devi KP, Suganthy N, Kesika P, Pandian SK. Bioprotective properties of seaweeds: in vitro evaluation of antioxidant activity and antimicrobial activity against food borne bacteria in relation to polyphenolic content. BMC Complement Altern Med. 2008; 8:38. PMID: 18613983.
Article16. Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008; 57:259–265. PMID: 18387817.
Article17. Sugawara T, Baskaran V, Tsuzuki W, Nagao A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J Nutr. 2002; 132:946–951. PMID: 11983819.
Article18. Ye J, Li Y, Teruya K, Katakura Y, Ichikawa A, Eto H, Hosoi M, Hosoi M, Nishimoto S, Shirahata S. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology. 2005; 47:117–126. PMID: 19003051.
Article19. Dias PF, Siqueira JM Jr, Maraschin M, Ferreira AG, Gagliardi AR, Ribeiro-do-Valle RM. A polysaccharide isolated from the brown seaweed Sargassum stenophyllum exerts antivasculogenic effects evidenced by modified morphogenesis. Microvasc Res. 2008; 75:34–44. PMID: 17585952.
Article20. Guerra Dore CM, Faustino Alves MG, Santos ND, Cruz AK, Câmara RB, Castro AJ, Guimarães Alves L, Nader HB, Leite EL. Antiangiogenic activity and direct antitumor effect from a sulfated polysaccharide isolated from seaweed. Microvasc Res. 2013; 88:12–18. PMID: 23507505.
Article21. Matsubara K, Mori M, Matsumoto H, Hori K, Miyazawa K. Antiangiogenic properties of a sulfated galactan isolated from a marine green alga, Codium cylindricum. J Appl Phycol. 2003; 15:87–90.22. Matsubara K, Xue C, Zhao X, Mori M, Sugawara T, Hirata T. Effects of middle molecular weight fucoidans on in vitro and ex vivo angiogenesis of endothelial cells. Int J Mol Med. 2005; 15:695–699. PMID: 15754034.
Article23. Vinayak RC, Sabu AS, Chatterji A. Bio-evaluation of two red seaweeds for their cytotoxic and antioxidant activities in vitro. J Complement Integr Med. 2010; 7:doi: 10.2202/1553-3840.1444.
Article24. Vinayak RC, Sabu AS, Chatterji A. Bio-prospecting of a few brown seaweeds for their cytotoxic and antioxidant activities. Evid Based Complement Alternat Med. 2011; 2011:673083. PMID: 21860651.
Article25. Vinayak RC, Sudha SA, Chatterji A. Bio-screening of a few green seaweeds from India for their cytotoxic and antioxidant potential. J Sci Food Agric. 2011; 91:2471–2476. PMID: 21674507.
Article26. Naderali EK, Brown MJ, Pickavance LC, Wilding JP, Doyle PJ, Williams G. Dietary obesity in the rat induces endothelial dysfunction without causing insulin resistance: a possible role for triacylglycerols. Clin Sci (Lond). 2001; 101:499–506. PMID: 11672455.
Article27. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007; 2:329–333. PMID: 17406593.
Article28. Gururaj AE, Belakavadi M, Salimath BP. Antiangiogenic effects of butyric acid involve inhibition of VEGF/KDR gene expression and endothelial cell proliferation. Mol Cell Biochem. 2003; 243:107–112. PMID: 12619895.29. Sarayba MA, Li L, Tungsiripat T, Liu NH, Sweet PM, Patel AJ, Osann KE, Chittiboyina A, Benson SC, Pershadsingh HA, Chuck RS. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-gamma ligand. Exp Eye Res. 2005; 80:435–442. PMID: 15721625.30. Sheela ML, Ramakrishna MK, Salimath BP. Angiogenic and proliferative effects of the cytokine VEGF in Ehrlich ascites tumor cells is inhibited by Glycyrrhiza glabra. Int Immunopharmacol. 2006; 6:494–498. PMID: 16428085.
Article31. Deepak AV, Salimath BP. Antiangiogenic and proapoptotic activity of a novel glycoprotein from U. indica is mediated by NF-kappaB and Caspase activated DNase in ascites tumor model. Biochimie. 2006; 88:297–307. PMID: 16216405.32. Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000; 105:R15–R24. PMID: 10772661.
Article33. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002; 2:727–739. PMID: 12360276.
Article34. Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O'Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000; 60:1878–1886. PMID: 10766175.35. Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol. 2001; 19:1195–1206. PMID: 11181686.
Article36. Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. J Clin Invest. 2000; 105:1045–1047. PMID: 10772648.
Article37. Kotnala S, Garg A, Chatterji A. Screening for the presence of antimicrobial activity in few Indian seaweeds. Pertanika J Trop Agric Sci. 2009; 32:69–75.39. Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat Rec. 2001; 264:317–324. PMID: 11745087.
Article40. Borgström P, Hillan KJ, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996; 56:4032–4039. PMID: 8752175.41. Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993; 362:841–844. PMID: 7683111.
Article42. Mesiano S, Ferrara N, Jaffe RB. Role of vascular endothelial growth factor in ovarian cancer: inhibition of ascites formation by immunoneutralization. Am J Pathol. 1998; 153:1249–1256. PMID: 9777956.43. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990; 82:4–6. PMID: 1688381.
Article44. Sithranga Boopathy N, Kathiresan K. Anticancer drugs from marine flora: an overview. J Oncol. 2010; 2010:214186. PMID: 21461373.
Article45. Birner P, Obermair A, Schindl M, Kowalski H, Breitenecker G, Oberhuber G. Selective immunohistochemical staining of blood and lymphatic vessels reveals independent prognostic influence of blood and lymphatic vessel invasion in early-stage cervical cancer. Clin Cancer Res. 2001; 7:93–97. PMID: 11205924.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Keratoacanthoma Centrifugum Marginatum
- Anti-inflammatory, Anti-arthritic and Analgesic Effect of the Herbal Extract Made from Bacopa monnieriis, Cassia fistula and Phyllanthus polyphyllus
- Anti-inflammatory effects of ethanolic extract of Annona muricata
- Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines
- Antioxidative Activity and Anti-melanogenic Effect of the Extract from the Leaves of Robinia Pseudo-acacia L