Nutr Res Pract.
2014 Jun;8(3):267-271.
The anti-inflammatory effect of Indonesian Areca catechu leaf extract in vitro and in vivo
- Affiliations
-
- 1Department of Medical Science, School of Medicine Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, Korea. hhong@kku.ac.kr
- 2Department of Pharmacology and Phytochemistry, Faculty of Pharmacy, Airlangga University, Indonesia.
- 3College of Animal Bioscience & Technology, Konkuk University, Seoul 143-701, Korea.
Abstract
- BACKGROUND/OBJECTIVES
Overproduction of nitric oxide (NO) by the inducible nitric oxide synthase (iNOS) enzyme can cause inflammation. Cyclooxygenase-2 (COX-2) is also involved in the inflammatory response through regulation of nuclear factor-kappa B (NF-kappaB). Areca catechu is one of the known fruit plants of the Palmaceae family. It has been used for a long time as a source of herbal medicine in Indonesia. In this study, we explored the effect of Indonesian Areca catechu leaf ethanol extract (ACE) in lipopolysaccharide (LPS)-induced inflammation and carrageenan-induced paw edema models. Recently, this natural extract has been in the spotlight because of its efficacy and limited or no toxic side effects. However, the mechanism underlying its anti-inflammatory effect remains to be elucidated.
MATERIALS/METHODS
We measured NO production by using the Griess reagent, and determined the expression levels of inflammation-related proteins, such as iNOS, COX2, and NF-kappaB, by western blot. To confirm the effect of ACE in vivo, we used the carrageenan-induced paw edema model.
RESULTS
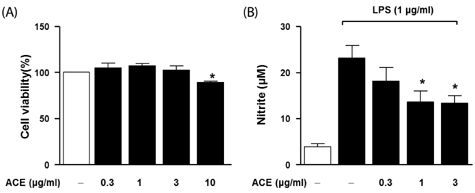
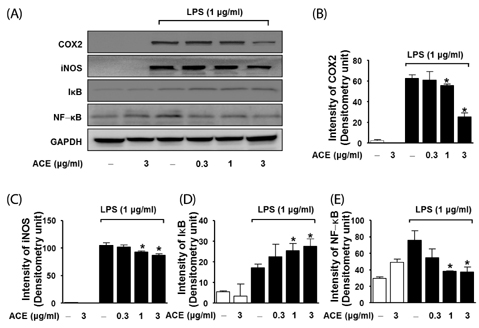
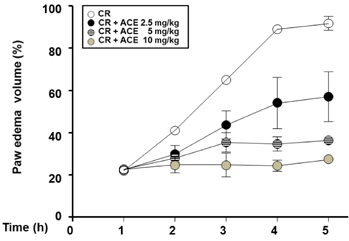
Compared to untreated cells, LPS-stimulated RAW 264.7 cells treated with ACE showed reduced NO generation and reduced iNOS and COX-2 expression. We found that the acute inflammatory response was significantly reduced by ACE in the carrageenan-induced paw edema model.
CONCLUSION
Taken together, these results suggest that ACE can inhibit inflammation and modulate NO generation via downregulation of iNOS levels and NF-kappaB signaling in vitro and in vivo. ACE may have a potential medical benefit as an anti-inflammation agent.
Keyword
MeSH Terms
Figure
Reference
-
1. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17:4–12.2. Kozel NJ, Adams EH. Epidemiology of drug abuse: an overview. Science. 1986; 234:970–974.
Article3. Leem KH, Park HK. Traditional Korean medicine: now and the future. Neurol Res. 2007; 29:Suppl 1. S3–S4.
Article4. Wang X, Zhang A, Sun H. Future perspectives of Chinese medical formulae: chinmedomics as an effector. OMICS. 2012; 16:414–421.
Article5. Salatino A, Fernandes-Silva CC, Righi AA, Salatino ML. Propolis research and the chemistry of plant products. Nat Prod Rep. 2011; 28:925–936.
Article6. Trusheva B, Popova M, Koendhori EB, Tsvetkova I, Naydenski C, Bankova V. Indonesian propolis: chemical composition, biological activity and botanical origin. Nat Prod Res. 2011; 25:606–613.
Article7. Khan S, Mehmood MH, Ali AN, Ahmed FS, Dar A, Gilani AH. Studies on anti-inflammatory and analgesic activities of betel nut in rodents. J Ethnopharmacol. 2011; 135:654–661.
Article8. Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011; 243:174–190.
Article9. Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007; 65:S140–S146.
Article10. Clària J, González-Périz A, López-Vicario C, Rius B, Titos E. New insights into the role of macrophages in adipose tissue inflammation and Fatty liver disease: modulation by endogenous omega-3 Fatty Acid-derived lipid mediators. Front Immunol. 2011; 2:49.
Article11. Walsh LJ. Mast cells and oral inflammation. Crit Rev Oral Biol Med. 2003; 14:188–198.
Article12. Li C, Wang MH. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr Res Pract. 2011; 5:101–106.
Article13. Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005; 1056:218–233.14. Park CM, Song YS. Luteolin and luteolin-7-O-glucoside inhibit lipopolysaccharide-induced inflammatory responses through modulation of NF-κB/AP-1/PI3K-Akt signaling cascades in RAW 264.7 cells. Nutr Res Pract. 2013; 7:423–429.
Article15. Halici Z, Dengiz GO, Odabasoglu F, Suleyman H, Cadirci E, Halici M. Amiodarone has anti-inflammatory and anti-oxidative properties: an experimental study in rats with carrageenan-induced paw edema. Eur J Pharmacol. 2007; 566:215–221.
Article16. Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000; 6:347–373.
Article17. Fereidoni M, Ahmadiani A, Semnanian S, Javan M. An accurate and simple method for measurement of paw edema. J Pharmacol Toxicol Methods. 2000; 43:11–14.
Article18. Elliott S, Brimacombe J. The medicinal plants of Gunung Leuser National Park, Indonesia. J Ethnopharmacol. 1987; 19:285–317.
Article19. O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997; 20:252–258.20. Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004; 3:401–416.
Article21. Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, Liou S, Simmons D, Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992; 267:25934–25938.
Article22. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998; 16:225–260.
Article23. Gao Y, Jiang W, Dong C, Li C, Fu X, Min L, Tian J, Jin H, Shen J. Anti-inflammatory effects of sophocarpine in LPS-induced RAW 264.7 cells via NF-κB and MAPKs signaling pathways. Toxicol In Vitro. 2012; 26:1–6.
Article24. Huo M, Cui X, Xue J, Chi G, Gao R, Deng X, Guan S, Wei J, Soromou LW, Feng H, Wang D. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J Surg Res. 2013; 180:e47–e54.
Article25. Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996; 87:13–20.26. Baldwin AS Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996; 14:649–683.27. Lin SC, Lu SY, Lee SY, Lin CY, Chen CH, Chang KW. Areca (betel) nut extract activates mitogen-activated protein kinases and NF-kappaB in oral keratinocytes. Int J Cancer. 2005; 116:526–535.
Article28. Huang PL, Chi CW, Liu TY. Effects of Areca catechu L. containing procyanidins on cyclooxygenase-2 expression in vitro and in vivo. Food Chem Toxicol. 2010; 48:306–313.
Article29. Vinegar R, Truax JF, Selph JL, Voelker FA. Pathway of onset, development, and decay of carrageenan pleurisy in the rat. Fed Proc. 1982; 41:2588–2595.30. Barman MR, Uddin MS, Akhter S, Ahmed MN, Haque Z, Rahman S, Mostafa F, Zaman M, Noor FA, Rahmatullah M. Antinociceptive activity of methanol extract of Areca catechu L.(Arecaceae) stems and leaves in mice. Adv Nat Appl Sci. 2011; 5:223–226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-inflammatory effects of ethanolic extract of Annona muricata
- Anticancer Effect of Persimmon Leaf Extracts on Korean Gastric Cancer Cell
- Erratum: Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- Antioxidant, anti-inflammatory, and anti-fibrotic properties of olive leaf extract protect against L-arginine induced chronic pancreatitis in the adult male albino rat