Nutr Res Pract.
2014 Apr;8(2):220-226.
Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats
- Affiliations
-
- 1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China. zzgneau05@aliyun.com
- 2School of Life Sciences, Inner Mongolia University for Nationalities, Tongliao 028000, China.
Abstract
- BACKGROUD/OBEJECTIVES: Arsenic, which causes human carcinogenicity, is ubiquitous in the environment. This study was designed to evaluate modulation of arsenic induced cancer by resveratrol, a phytoalexin found in vegetal dietary sources that has antioxidant and chemopreventive properties, in arsenic trioxide (As2O3)-induced Male Wistar rats.
MATERIALS/METHODS
Adult rats received 3 mg/kg As2O3 (intravenous injection, iv.) on alternate days for 4 days. Resveratrol (8 mg/kg) was administered (iv.) 1 h before As2O3 treatment. The plasma and homogenization enzymes associated with oxidative stress of rat kidneys were measured, the kidneys were examined histologically and trace element contents were assessed.
RESULTS
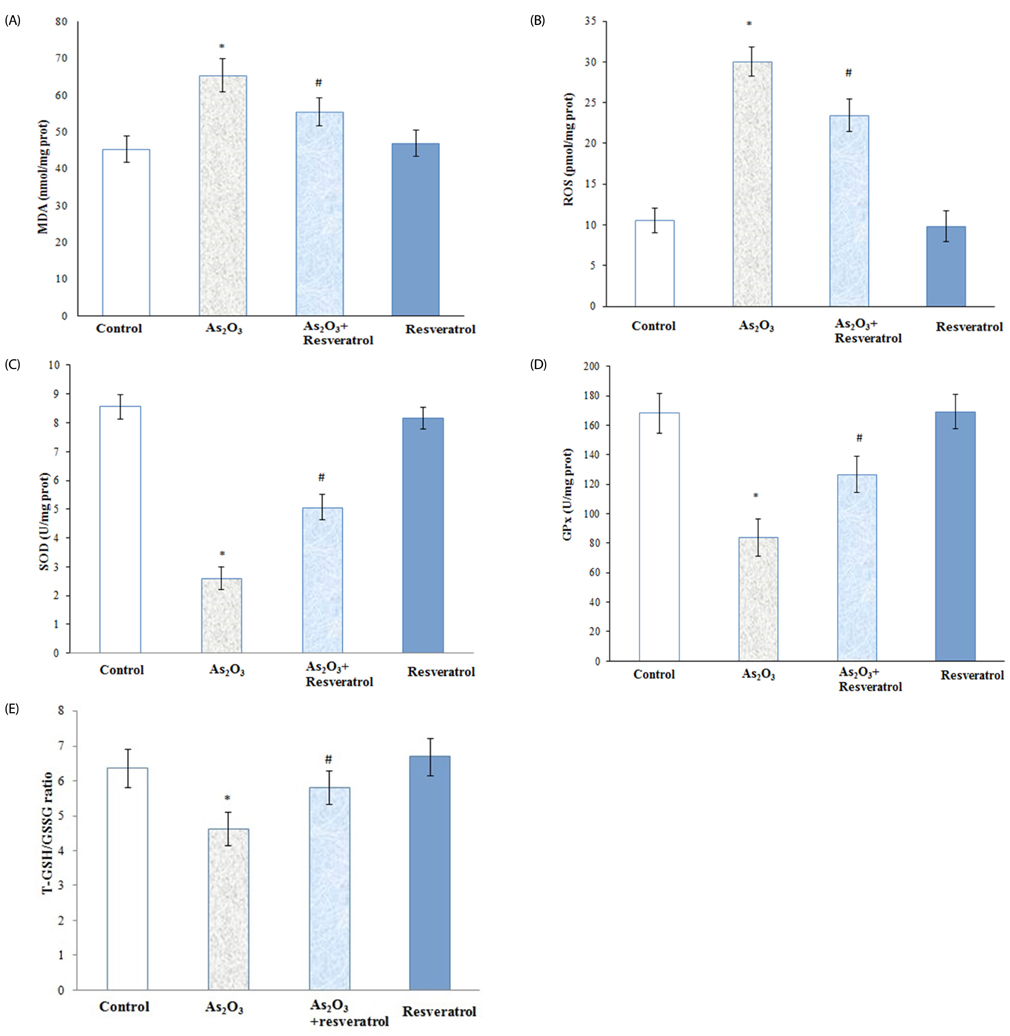
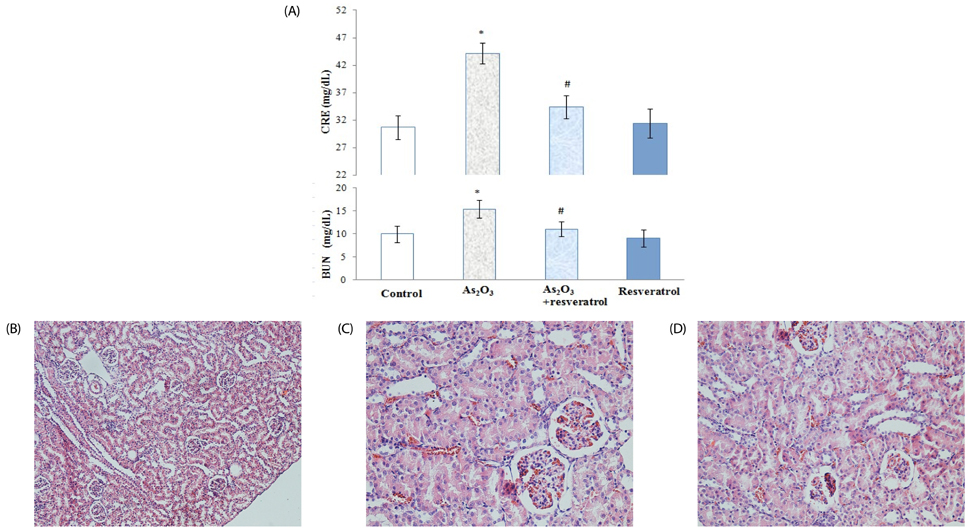
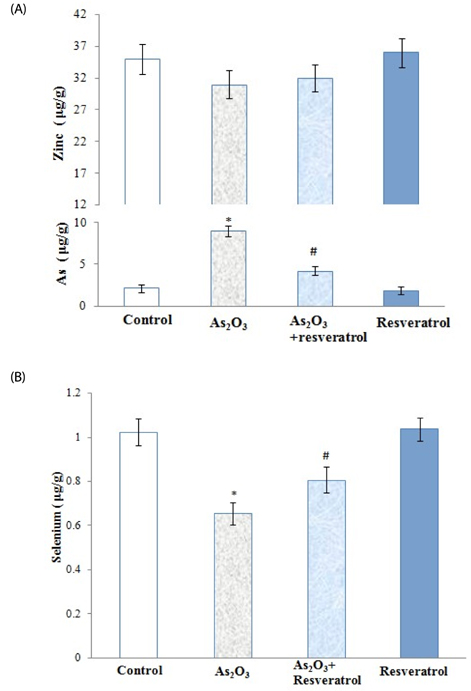
Rats treated with As2O3 had significantly higher oxidative stress and kidney arsenic accumulation; however, pretreatment with resveratrol reversed these changes. In addition, prior to treatment with resveratrol resulted in lower blood urea nitrogen, creatinine and insignificant renal tubular epithelial cell necrosis. Furthermore, the presence of resveratrol preserved the selenium content (0.805 +/- 0.059 microg/g) of kidneys in rats treated with As2O3. However, resveratrol had no effect on zinc level in the kidney relative to As2O3-treated groups.
CONCLUSIONS
Our data show that supplementation with resveratrol alleviated nephrotoxicity by improving antioxidant capacity and arsenic efflux. These findings suggest that resveratrol has the potential to protect against kidney damage in populations exposed to arsenic.
Keyword
MeSH Terms
Figure
Reference
-
1. Yu M, Xue J, Li Y, Zhang W, Ma D, Liu L, Zhang Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch Toxicol. 2013; 87:1025–1035.
Article2. Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Arsenic exposure and toxicology: a historical perspective. Toxicol Sci. 2011; 123:305–332.
Article3. Singh S, Mulchandani A, Chen W. Highly selective and rapid arsenic removal by metabolically engineered Escherichia coli cells expressing fucus vesiculosus metallothionein. Appl Environ Microbiol. 2008; 74:2924–2927.
Article4. Wang L, Kou MC, Weng CY, Hu LW, Wang YJ, Wu MJ. Arsenic modulates heme oxygenase-1, interleukin-6, and vascular endothelial growth factor expression in endothelial cells: roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol. 2012; 86:879–896.
Article5. Li Z, Piao F, Liu S, Wang Y, Qu S. Subchronic exposure to arsenic trioxide-induced oxidative DNA damage in kidney tissue of mice. Exp Toxicol Pathol. 2010; 62:543–547.
Article6. Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006; 5:493–506.
Article7. Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005; 211:26–35.
Article8. Dudka J, Gieroba R, Korga A, Burdan F, Matysiak W, Jodlowska-Jedrych B, Mandziuk S, Korobowicz E, Murias M. Different effects of resveratrol on dose-related Doxorubicin-induced heart and liver toxicity. Evid Based Complement Alternat Med. 2012; 2012:606183.
Article9. Gülçin İ. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012; 86:345–391.
Article10. Csanaky I, Gregus Z. Effect of selenite on the disposition of arsenate and arsenite in rats. Toxicology. 2003; 186:33–50.
Article11. Kucukatay V, Turgut S, Kocamaz E, Emmungil G, Bor-Kucukatay M, Turgut G, Akca H, Bagci H. Effect of sulfite exposure on zinc, iron, and copper levels in rat liver and kidney tissues. Biol Trace Elem Res. 2006; 114:185–195.
Article12. Entwisle J, Hearn R. Development of an accurate procedure for the determination of arsenic in fish tissues of marine origin by inductively coupled plasma mass spectrometry. Spectrochim Acta Part B At Spectrosc. 2006; 61:438–443.
Article13. Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007; 224:274–283.
Article14. Scott E, Steward WP, Gescher AJ, Brown K. Resveratrol in human cancer chemoprevention--choosing the 'right' dose. Mol Nutr Food Res. 2012; 56:7–13.15. Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007; 16:1246–1252.
Article16. Zhang W, Xue J, Ge M, Yu M, Liu L, Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol. 2013; 51:87–92.
Article17. Lu KH, Lee HJ, Huang ML, Lai SC, Ho YL, Chang YS, Chi CW. Synergistic apoptosis-inducing antileukemic effects of arsenic trioxide and mucuna macrocarpa stem extract in human leukemic cells via a reactive oxygen species-dependent mechanism. Evid Based Complement Alternat Med. 2012; 2012:921430.18. Rodrigo R, Rivera G. Renal damage mediated by oxidative stress: a hypothesis of protective effects of red wine. Free Radic Biol Med. 2002; 33:409–422.
Article19. Han JY, Ahn SY, Oh EH, Nam SY, Hong JT, Oh KW, Lee MK. Red ginseng extract attenuates kainate-induced excitotoxicity by antioxidative effects. Evid Based Complement Alternat Med. 2012; 2012:479016.
Article20. Maiti S, Chatterjee AK. Effects on levels of glutathione and some related enzymes in tissues after an acute arsenic exposure in rats and their relationship to dietary protein deficiency. Arch Toxicol. 2001; 75:531–537.
Article21. Kang NE, Ha AW, Kim JY, Kim WK. Resveratrol inhibits the protein expression of transcription factors related adipocyte differentiation and the activity of matrix metalloproteinase in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2012; 6:499–504.
Article22. Lee HS, Ha AW, Kim WK. Effect of resveratrol on the metastasis of 4T1 mouse breast cancer cells in vitro and in vivo. Nutr Res Pract. 2012; 6:294–300.
Article23. Misbahuddin M, Islam AZ, Khandker S, Ifthaker Al M, Islam N, Anjumanara . Efficacy of spirulina extract plus zinc in patients of chronic arsenic poisoning: a randomized placebo-controlled study. Clin Toxicol (Phila). 2006; 44:135–141.
Article24. Pal S, Chatterjee AK. Protective effect of N-acetylcysteine against arsenic-induced depletion in vivo of carbohydrate. Drug Chem Toxicol. 2004; 27:179–189.
Article25. Flora SJ, Kannan GM, Kumar P. Selenium effects on gallium arsenide induced biochemical and immunotoxicological changes in rats. Chem Biol Interact. 1999; 122:1–13.
Article26. Anzai N, Endou H. World Congress on Alternatives and Animal Use in the Life Sciences. Renal drug transporters and nephrotoxicity. In : Review Progress Made toward the 3Rs : Proceedings / 6th World Congress on Alternatives & Animal Use in the Life Sciences; Tokyo: Japanese Society for Alternatives to Animal Experiments;2008. p. 447–452.27. Johnson CH, Patterson AD, Idle JR, Gonzalez FJ. Xenobiotic metabolomics: major impact on the metabolome. Annu Rev Pharmacol Toxicol. 2012; 52:37–56.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats
- Osteomyelitis on Maxilla Caused by Arsenic Trioxide
- Antitumor Effects of Arsenic Trioxide on Neuroblastoma
- A Case of Relapsed Acute Promyleocytic Leukemia Induced Remission with Arsenic Trioxide(As2O3)
- Arsenic Trioxide, an Old Drug? or a New Drug?