Nutr Res Pract.
2014 Feb;8(1):46-53.
Nephroprotective effect of astaxanthin against trivalent inorganic arsenic-induced renal injury in wistar rats
- Affiliations
-
- 1College of Veterinary Medicine, Northeast Agricultural University, Harbin 150030, China. zhangzhigang@neau.edu.cn
Abstract
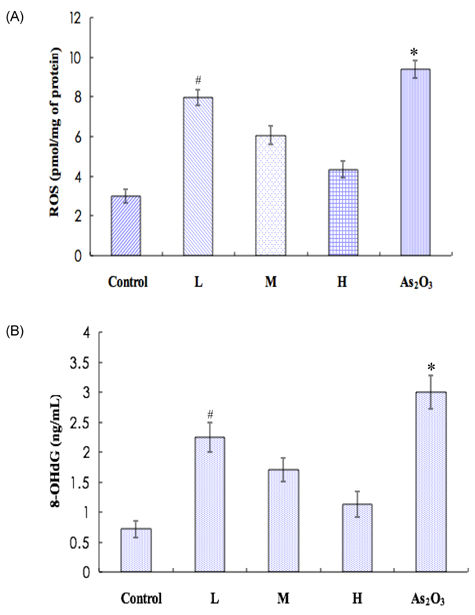
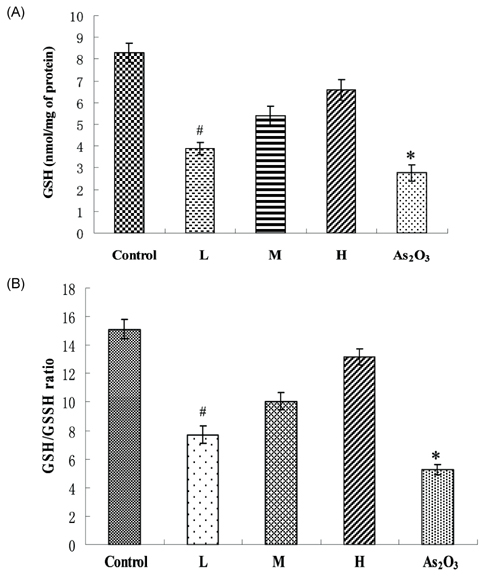
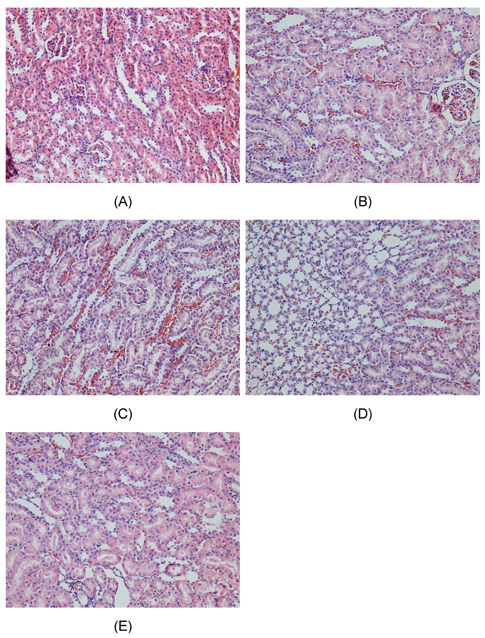
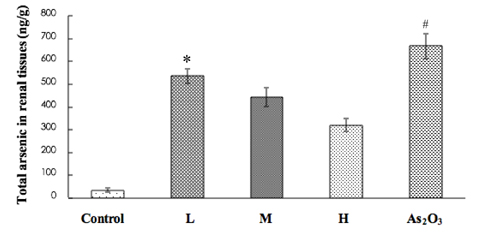
- Inorganic arsenic (iAs) is a toxic metalloid found ubiquitously in the environment. In humans, exposure to iAs can result in toxicity and cause toxicological manifestations. Arsenic trioxide (As2O3) has been used in the treatment for acute promyelocytic leukemia. The kidney is the critical target organ of trivalent inorganic As (iAsIII) toxicity. We examine if oral administration of astaxanthin (AST) has protective effects on nephrotoxicity and oxidative stress induced by As2O3 exposure (via intraperitoneal injection) in rats. Markers of renal function, histopathological changes, Na+-K+ ATPase, sulfydryl, oxidative stress, and As accumulation in kidneys were evaluated as indicators of As2O3 exposure. AST showed a significant protective effect against As2O3-induced nephrotoxicity. These results suggest that the mechanisms of action, by which AST reduces nephrotoxicity, may include antioxidant protection against oxidative injury and reduction of As accumulation. These findings might be of therapeutic benefit in humans or animals suffering from exposure to iAsIII from natural sources or cancer therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Mortensen A, Skibsted LH, Truscott TG. The interaction of dietary carotenoids with radical species. Arch Biochem Biophys. 2001; 385:13–19.
Article2. Liu X, Osawa T. Astaxanthin protects neuronal cells against oxidative damage and is a potent candidate for brain food. Forum Nutr. 2009; 61:129–135.
Article3. Lennikov A, Kitaichi N, Fukase R, Murata M, Noda K, Ando R, Ohguchi T, Kawakita T, Ohno S, Ishida S. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops. Mol Vis. 2012; 18:455–464.4. Baig JA, Kazi TG, Shah AQ, Afridi HI, Kandhro GA, Khan S, Kolachi NF, Wadhwa SK, Shah F, Arain MB, Jamali MK. Evaluation of arsenic levels in grain crops samples, irrigated by tube well and canal water. Food Chem Toxicol. 2011; 49:265–270.
Article5. Horner NS, Beauchemin D. The effect of cooking and washing rice on the bio-accessibility of As, Cu, Fe, V and Zn using an on-line continuous leaching method. Anal Chim Acta. 2013; 758:28–35.
Article6. Hughes MF. Arsenic toxicity and potential mechanisms of action. Toxicol Lett. 2002; 133:1–16.
Article7. Yen YP, Tsai KS, Chen YW, Huang CF, Yang RS, Liu SH. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch Toxicol. 2012; 86:923–933.
Article8. Henkler F, Brinkmann J, Luch A. The role of oxidative stress in carcinogenesis induced by metals and xenobiotics. Cancer. 2010; 2:376–396.
Article9. Dwivedi N, Flora SJ. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem Toxicol. 2011; 49:1152–1159.
Article10. Varol E, Varol S. Effect of fluoride toxicity on cardiovascular systems: role of oxidative stress. Arch Toxicol. 2012; 86:1627.
Article11. Das TK, Mani V, De S, Banerjee D, Mukherjee A, Polley S, Kewalramani N, Kaur H. Effect of vitamin E supplementation on mRNA expression of superoxide dismutase and interleukin-2 in arsenic exposed goat leukocytes. Bull Environ Contam Toxicol. 2012; 89:1133–1137.
Article12. Zhang W, Xue J, Ge M, Yu M, Liu L, Zhang Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem Toxicol. 2013; 51:87–92.
Article13. Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem. 2000; 48:1150–1154.
Article14. Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990; 22:27–38.15. Kosanić M, Manojlović N, Janković S, Stanojković T, Ranković B. Evernia prunastri and Pseudoevernia furfuraceae lichens and their major metabolites as antioxidant, antimicrobial and anticancer agents. Food Chem Toxicol. 2013; 53:112–118.
Article16. Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutré S, Dahlberg S, Ellison R, Warrell RP Jr. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001; 19:3852–3860.
Article17. Zhang XW, Yan XJ, Zhou ZR, Yang FF, Wu ZY, Sun HB, Liang WX, Song AX, Lallemand-Breitenbach V, Jeanne M, Zhang QY, Yang HY, Huang QH, Zhou GB, Tong JH, Zhang Y, Wu JH, Hu HY, de The H, Chen SJ, Chen Z. Arsenic trioxide controls the fate of the PML-RARalpha oncoprotein by directly binding PML. Science. 2010; 328:240–243.
Article18. Chen SJ, Zhou GB, Zhang XW, Mao JH, de Thé H, Chen Z. From an old remedy to a magic bullet: molecular mechanisms underlying the therapeutic effects of arsenic in fighting leukemia. Blood. 2011; 117:6425–6437.
Article19. Nandi D, Patra RC, Swarup D. Effect of cysteine, methionine, ascorbic acid and thiamine on arsenic-induced oxidative stress and biochemical alterations in rats. Toxicology. 2005; 211:26–35.
Article20. Yousef MI, El-Demerdash FM, Radwan FM. Sodium arsenite induced biochemical perturbations in rats: ameliorating effect of curcumin. Food Chem Toxicol. 2008; 46:3506–3511.
Article21. Augusti PR, Conterato GM, Somacal S, Sobieski R, Spohr PR, Torres JV, Charão MF, Moro AM, Rocha MP, Garcia SC, Emanuelli T. Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem Toxicol. 2008; 46:212–219.
Article22. Curek GD, Cort A, Yucel G, Demir N, Ozturk S, Elpek GO, Savas B, Aslan M. Effect of astaxanthin on hepatocellular injury following ischemia/reperfusion. Toxicology. 2010; 267:147–153.
Article23. Lee DH, Kim CS, Lee YJ. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem Toxicol. 2011; 49:271–280.
Article24. Cui X, Li S, Shraim A, Kobayashi Y, Hayakawa T, Kanno S, Yamamoto M, Hirano S. Subchronic exposure to arsenic through drinking water alters expression of cancer-related genes in rat liver. Toxicol Pathol. 2004; 32:64–72.
Article25. Lakritz J, Plopper CG, Buckpitt AR. Validated high-performance liquid chromatography-electrochemical method for determination of glutathione and glutathione disulfide in small tissue samples. Anal Biochem. 1997; 247:63–68.
Article26. Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol. 1999; 155:109–117.
Article27. Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011; 74:607–614.
Article28. Stewart JS, Lignell A, Pettersson A, Elfving E, Soni MG. Safety assessment of astaxanthin-rich microalgae biomass: acute and subchronic toxicity studies in rats. Food Chem Toxicol. 2008; 46:3030–3036.
Article29. Petri D, Lundebye AK. Tissue distribution of astaxanthin in rats following exposure to graded levels in the feed. Comp Biochem Physiol C Toxicol Pharmacol. 2007; 145:202–209.
Article30. Trpkovic A, Todorovic-Markovic B, Trajkovic V. Toxicity of pristine versus functionalized fullerenes: mechanisms of cell damage and the role of oxidative stress. Arch Toxicol. 2012; 86:1809–1827.
Article31. Oguri T, Yoshinaga J, Tao H, Nakazato T. Daily intake of inorganic arsenic and some organic arsenic species of Japanese subjects. Food Chem Toxicol. 2012; 50:2663–2667.
Article32. Chen Y, Krishan M, Nebert DW, Shertzer HG. Glutathione-deficient mice are susceptible to TCDD-induced hepatocellular toxicity but resistant to steatosis. Chem Res Toxicol. 2012; 25:94–100.
Article33. Bhalla S, Gordon LI, David K, Prachand S, Singh AT, Yang S, Winter JN, Guo D, O'Halloran T, Platanias LC, Evens AM. Glutathione depletion enhances arsenic trioxide-induced apoptosis in lymphoma cells through mitochondrial-independent mechanisms. Br J Haematol. 2010; 150:365–369.
Article34. Qin XJ, Hudson LG, Liu W, Ding W, Cooper KL, Liu KJ. Dual actions involved in arsenite-induced oxidative DNA damage. Chem Res Toxicol. 2008; 21:1806–1813.
Article35. Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, Hudson LG. Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs. J Biol Chem. 2011; 286:22855–22863.
Article36. Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010; 123:2533–2542.
Article37. Cheng B, Yang X, An L, Gao B, Liu X. Arsenic trioxide-induced apoptosis of Hep-2 cell line through modulating intracellular glutathione (GSH) level. Auris Nasus Larynx. 2010; 37:89–94.
Article38. Wolf AM, Asoh S, Hiranuma H, Ohsawa I, Iio K, Satou A, Ishikura M, Ohta S. Astaxanthin protects mitochondrial redox state and functional integrity against oxidative stress. J Nutr Biochem. 2010; 21:381–389.
Article39. Marafante E, Vahter M, Envall J. The role of the methylation in the detoxication of arsenate in the rabbit. Chem Biol Interact. 1985; 56:225–238.
Article40. Healy SM, Casarez EA, Ayala-Fierro F, Aposhian H. Enzymatic methylation of arsenic compounds. V. Arsenite methyltransferase activity in tissues of mice. Toxicol Appl Pharmacol. 1998; 148:65–70.41. Lin S, Shi Q, Nix FB, Styblo M, Beck MA, Herbin-Davis KM, Hall LL, Simeonsson JB, Thomas DJ. A novel S-adenosyl-L-methionine: arsenic(III) methyltransferase from rat liver cytosol. J Biol Chem. 2002; 277:10795–10803.
Article42. Leslie EM. Arsenic-glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs). J Inorg Biochem. 2012; 108:141–149.
Article43. Chiou HY, Hsueh YM, Hsieh LL, Hsu LI, Hsu YH, Hsieh FI, Wei ML, Chen HC, Yang HT, Leu LC, Chu TH, Chen-Wu C, Yang MH, Chen CJ. Arsenic methylation capacity, body retention, and null genotypes of glutathione S-transferase M1 and T1 among current arsenic-exposed residents in Taiwan. Mutat Res. 1997; 386:197–207.
Article44. Emadi A, Gore SD. Arsenic trioxide - An old drug rediscovered. Blood Rev. 2010; 24:191–199.
Article45. Zhao L, Chen F, Zhao G, Wang Z, Liao X, Hu X. Isomerization of trans-astaxanthin induced by copper(II) ion in ethanol. J Agric Food Chem. 2005; 53:9620–9623.
Article46. Haque MS, Roy SK, Shahjahan M. Arsenic impairs the effect of low temperature on the regulation of Na+-K+ ATPase activity in skeletal muscle of fish (Channa punctata). Turkish J Fish Aquat Sci. 2011; 11:339–344.47. Stanton CR, Thibodeau R, Lankowski A, Shaw JR, Hamilton JW, Stanton BA. Arsenic inhibits CFTR-mediated chloride secretion by killifish (Fundulus heteroclitus) opercular membrane. Cell Physiol Biochem. 2006; 17:269–278.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats
- Assessment of Arsenic Exposure by Measurement of Urinary Speciated Inorganic Arsenic Metabolites in Workers in a Semiconductor Manufacturing Plant
- Acute Toxicity of Arsenic in Rats and Mice
- Astaxanthin Inhibits Helicobacter pylori-induced Inflammatory and Oncogenic Responses in Gastric Mucosal Tissues of Mice
- Health Effects of Chronic Arsenic Exposure