Nutr Res Pract.
2011 Oct;5(5):381-388.
Comparative effect of genistein and daidzein on the expression of MCP-1, eNOS, and cell adhesion molecules in TNF-alpha-stimulated HUVECs
- Affiliations
-
- 1Paik Institute for Clinical Research, Inje University, Busan 614-735, Korea.
- 2Department of Smart Foods and Drugs, Inje University, 607 Obang-dong, Gimhae, Gyeongnam 621-749, Korea. fdsnsong@inje.ac.kr
- 3Department of Food and Nutrition, Silla University, Busan 617-736, Korea.
- 4School of Biomedical and Biotechnology, Inje University, Gimhae, Gyeongnam 621-749, Korea.
Abstract
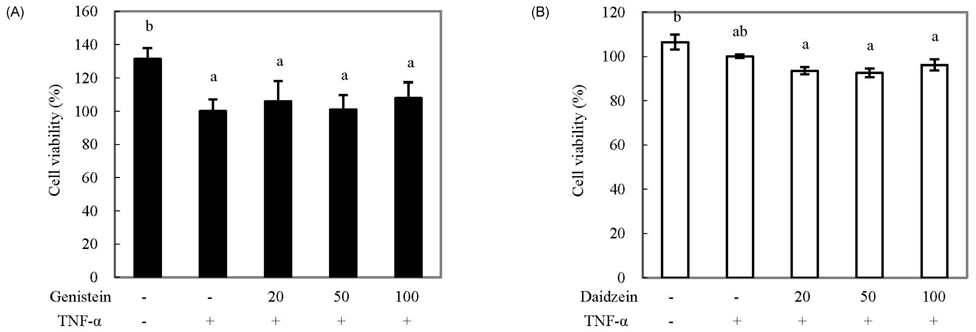
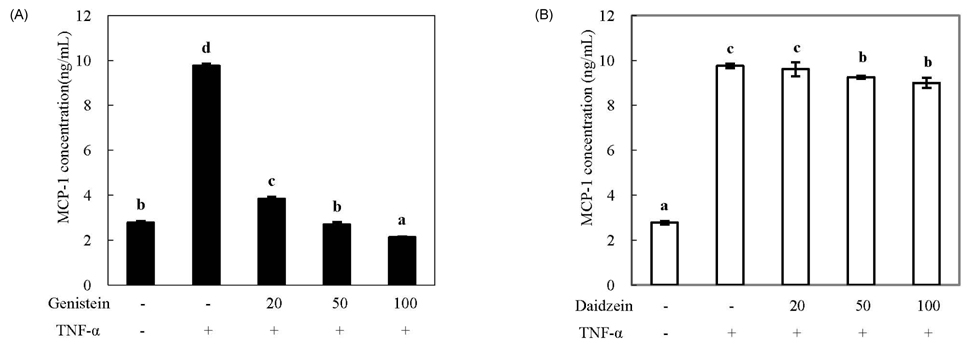
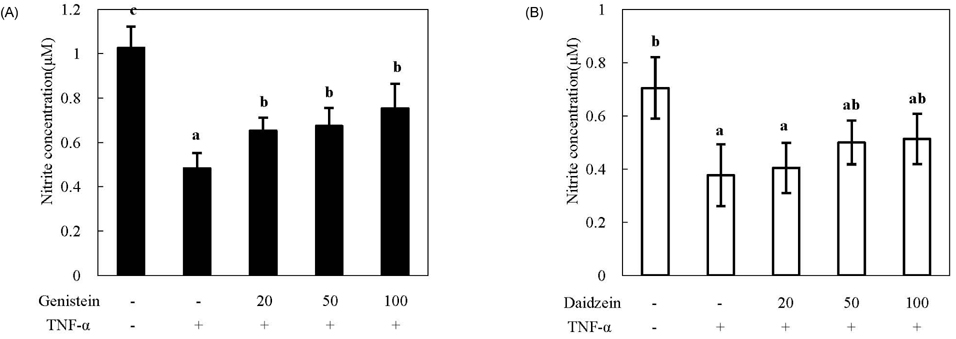
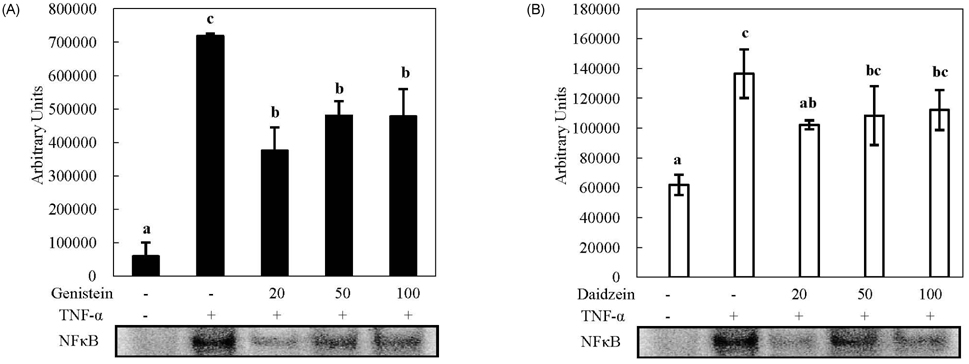
- We compared the effects of genistein and daidzein on the expression of chemokines, cell adhesion molecules (CAMs), and endothelial nitric oxide synthase (eNOS) in tumor necrosis factor (TNF)-alpha-stimulated human umbilical vascular endothelial cells (HUVECs). TNF-alpha exposure significantly increased expression of monocyte chemoattractant protein (MCP)-1, vascular adhesion molecule (VCAM)-1, and intercellular adhesion molecule-1. Genistein significantly decreased MCP-1 and VCAM-1 production in a dose-dependent manner, whereas CAM expression was not significantly lowered by genistein treatment. However, daidzein slightly decreased MCP-1 production. The effects of genistein and daidzein on MCP-1 secretion coincided with mRNA expression. Pre-treatment with either genistein or daidzein elevated eNOS expression and nitric oxide production disturbed by TNF-alpha exposure. A low concentration of isoflavones significantly inhibited nuclear factor (NF)kappaB activation, whereas a high dose slightly ameliorated these inhibitive effects. These results suggest that genistein had a stronger effect on MCP-1 and eNOS expression than that of daidzein. Additionally, NFkappaB transactivation might be partially related to the down-regulation of these mRNAs in TNF-alpha-stimulated HUVECs.
Keyword
MeSH Terms
-
Cell Adhesion
Cell Adhesion Molecules
Chemokines
Down-Regulation
Endothelial Cells
Genistein
Humans
Intercellular Adhesion Molecule-1
Isoflavones
Monocytes
Nitric Oxide
Nitric Oxide Synthase Type III
RNA, Messenger
Transcriptional Activation
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Cell Adhesion Molecules
Chemokines
Genistein
Intercellular Adhesion Molecule-1
Isoflavones
Nitric Oxide
Nitric Oxide Synthase Type III
RNA, Messenger
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
1. Anthony MS, Clarkson TB, Williams JK. Effects of soy isoflavones on atherosclerosis: potential mechanisms. Am J Clin Nutr. 1998. 68:1390S–1393S.
Article2. Anthony MS, Clarkson TB, Hughes CL Jr, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J Nutr. 1996. 126:43–50.
Article3. Lee YM, Jung MH, Lee YS, Song J. Effect of genistein and soy protein on lipids metabolism in ovariectomized rats. Korean J Nutr. 2005. 38:267–278.4. Kirk EA, Sutherland P, Wang SA, Chait A, LeBoeuf RC. Dietary isoflavones reduce plasma cholesterol and atherosclerosis in C57BL/6 mice but not LDL receptor-deficient mice. J Nutr. 1998. 128:954–959.
Article5. Vera R, Sánchez M, Galisteo M, Villar IC, Jimenez R, Zarzuelo A, Pérez-Vizcaíno F, Duarte J. Chronic administration of genistein improves endothelial dysfunction in spontaneously hypertensive rats: involvement of eNOS, caveolin and calmodulin expression and NADPH oxidase activity. Clin Sci (Lond). 2007. 112:183–191.
Article6. Mahn K, Borrás C, Knock GA, Taylor P, Khan IY, Sugden D, Poston L, Ward JP, Sharpe RM, Viña J, Aaronson PI, Mann GE. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005. 19:1755–1757.
Article7. Si H, Liu D. Genistein, a soy phytoestrogen, upregulates the expression of human endothelial nitric oxide synthase and lowers blood pressure in spontaneously hypertensive rats. J Nutr. 2008. 138:297–304.
Article8. Rimbach G, Boesch-Saadatmandi C, Frank J, Fuchs D, Wenzel U, Daniel H, Hall WL, Weinberg PD. Dietary isoflavones in the prevention of cardiovascular disease--a molecular perspective. Food Chem Toxicol. 2008. 46:1308–1319.
Article9. Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995. 91:2488–2496.10. Bowie AG, Moynagh PN, O'Neill LA. Lipid peroxidation is involved in the activation of NF-κB by tumor necrosis factor but not interleukin-1 in the human endothelial cell line ECV304. Lack of involvement of H2O2 in NF-κB activation by either cytokine in both primary and transformed endothelial cells. J Biol Chem. 1997. 272:25941–25950.
Article11. May MJ, Wheeler-Jones CP, Pearson JD. Effects of protein tyrosine kinase inhibitors on cytokine-induced adhesion molecule expression by human umbilical vein endothelial cells. Br J Pharmacol. 1996. 118:1761–1771.
Article12. Adamson P, Tighe M, Pearson JD. Protein tyrosine kinase inhibitors act downstream of IL-1 alpha and LPS stimulated MAP-kinase phosphorylation to inhibit expression of E-selectin on human umbilical vein endothelial cells. Cell Adhes Commun. 1996. 3:511–525.
Article13. Majewska E, Paleolog E, Baj Z, Kralisz U, Feldmann M, Tchórzewski H. Role of tyrosine kinase enzymes in TNF-alpha and IL-1 induced expression of ICAM-1 and VCAM-1 on human umbilical vein endothelial cells. Scand J Immunol. 1997. 45:385–392.
Article14. Lee YS, Jang SY, Kim KO. Effects of soy isoflavone intake on nitrite content and antioxidant enzyme activities in male rats fed high-fat diet. Korean J Nutr. 2005. 38:89–95.15. Liu D, Homan LL, Dillon JS. Genistein acutely stimulates nitric oxide synthesis in vascular endothelial cells by a cyclic adenosine 5'-monophosphate-dependent mechanism. Endocrinology. 2004. 145:5532–5539.
Article16. Yen GC, Lai HH. Inhibitory effects of isoflavones on nitric oxide- or peroxynitrite-mediated DNA damage in RAW 264.7 cells and phiX174 DNA. Food Chem Toxicol. 2002. 40:1433–1440.
Article17. Sheu F, Lai HH, Yen GC. Suppression effect of soy isoflavones on nitric oxide production in RAW 264.7 macrophages. J Agric Food Chem. 2001. 49:1767–1772.
Article18. Mizutani K, Ikeda K, Nishikata T, Yamori Y. Phytoestrogens attenuate oxidative DNA damage in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. J Hypertens. 2000. 18:1833–1840.
Article19. Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic Res. 1997. 26:63–70.
Article20. Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr Cancer. 1993. 20:1–12.
Article21. Kapiotis S, Hermann M, Held I, Seelos C, Ehringer H, Gmeiner BM. Genistein, the dietary-derived angiogenesis inhibitor, prevents LDL oxidation and protects endothelial cells from damage by atherogenic LDL. Arterioscler Thromb Vasc Biol. 1997. 17:2868–2874.
Article22. Sadowska-Krowicka H, Mannick EE, Oliver PD, Sandoval M, Zhang XJ, Eloby-Childess S, Clark DA, Miller MJ. Genistein and gut inflammation: role of nitric oxide. Proc Soc Exp Biol Med. 1998. 217:351–357.
Article23. Gottstein N, Ewins BA, Eccleston C, Hubbard GP, Kavanagh IC, Minihane AM, Weinberg PD, Rimbach G. Effect of genistein and daidzein on platelet aggregation and monocyte and endothelial function. Br J Nutr. 2003. 89:607–615.
Article24. Fautz R, Husein B, Hechenberger C. Application of the neutral red assay (NR assay) to monolayer cultures of primary hepatocytes: rapid colorimetric viability determination for the unscheduled DNA synthesis test (UDS). Mutat Res. 1991. 253:173–179.
Article25. D'Agostino P, Ferlazzo V, Milano S, La Rosa M, Di Bella G, Caruso R, Barbera C, Grimaudo S, Tolomeo M, Feo S, Cillari E. Anti-inflammatory effects of chemically modified tetracyclines by the inhibition of nitric oxide and interleukin-12 synthesis in J774 cell line. Int Immunopharmacol. 2001. 1:1765–1776.26. Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983. 11:1475–1489.
Article27. Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A. Phyto-oestrogens: where are we now? Br J Nutr. 1998. 79:393–406.
Article28. Weber C, Negrescu E, Erl W, Pietsch A, Frankenberger M, Ziegler-Heitbrock HW, Siess W, Weber PC. Inhibitors of protein tyrosine kinase suppress TNF-stimulated induction of endothelial cell adhesion molecules. J Immunol. 1995. 155:445–451.29. Libby P, Galis ZS. Cytokines regulate genes involved in atherogenesis. Ann N Y Acad Sci. 1994. 748:158–168.30. Chen H, Chen Y, Tian W, Lei S, Peng R. Effects of estradiol and isoflavoid on the expression of adhesion molecules on neutrophils. Hua Xi Yi Ke Da Xue Xue Bao. 2001. 32:27–31.31. Lund CO, Mortensen A, Nilas L, Breinholt VM, Larsen JJ, Ottesen B. Estrogen and phytoestrogens: Effect on eNOS expression and in vitro vasodilation in cerebral arteries in ovariectomized Watanabe heritable hyperlipidemic rabbits. Eur J Obstet Gynecol Reprod Biol. 2007. 130:84–92.
Article32. Woodman OL, Missen MA, Boujaoude M. Daidzein and 17 beta-estradiol enhance nitric oxide synthase activity associated with an increase in calmodulin and a decrease in caveolin-1. J Cardiovasc Pharmacol. 2004. 44:155–163.
Article33. Kim DS, Kwon HM, Choi JS, Kang SW, Ji GE, Kang YH. Resveratrol blunts tumor necrosis factor-α-induced monocyte adhesion and transmigration. Nutr Res Pract. 2007. 1:285–290.
Article34. Guo JM, Xiao BX, Liu DH, Grant M, Zhang S, Lai YF, Guo YB, Liu Q. Biphasic effect of daidzein on cell growth of human colon cancer cells. Food Chem Toxicol. 2004. 42:1641–1646.
Article35. Tetsuka T, Srivastava SK, Morrison AR. Tyrosine kinase inhibitors, genistein and herbimycin A, do not block interleukin-1 beta-induced activation of NF-kappa B in rat mesangial cells. Biochem Biophys Res Commun. 1996. 218:808–812.
Article36. Lee YW, Kühn H, Hennig B, Neish AS, Toborek M. IL-4-induced oxidative stress upregulates VCAM-1 gene expression in human endothelial cells. J Mol Cell Cardiol. 2001. 33:83–94.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Ultraviolet Light on the Expression of Adhesion Molecules and T Lymphocyte Adhesion to Human Dermal Microvascular Endothelial Cells
- Heparin Attenuates the Expression of TNF alpha-induced Cerebral Endothelial Cell Adhesion Molecule
- Curcumin Attenuates Nuclear Factor-kappaB, c-Jun N-Terminal Kinase and p38 in Tumor Necrosis Factor-alpha-Stimulated Endothelial Cells
- Effect of VCAM-1 expression in human endothelial cells by proinflammatory cytokines
- Effect of cytokines on the expression of cell adhesion molecule and on the adhesion of melanoma cells to endothelial cells