Korean J Physiol Pharmacol.
2008 Oct;12(5):231-236. 10.4196/kjpp.2008.12.5.231.
Heparin Attenuates the Expression of TNF alpha-induced Cerebral Endothelial Cell Adhesion Molecule
- Affiliations
-
- 1Department of Pharmacology, Brain Research Institute, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. ahnys@yuhs.ac
- 2Department of Pharmacology, Pochun CHA University College of Medicine, Pocheon, Korea.
- KMID: 1486104
- DOI: http://doi.org/10.4196/kjpp.2008.12.5.231
Abstract
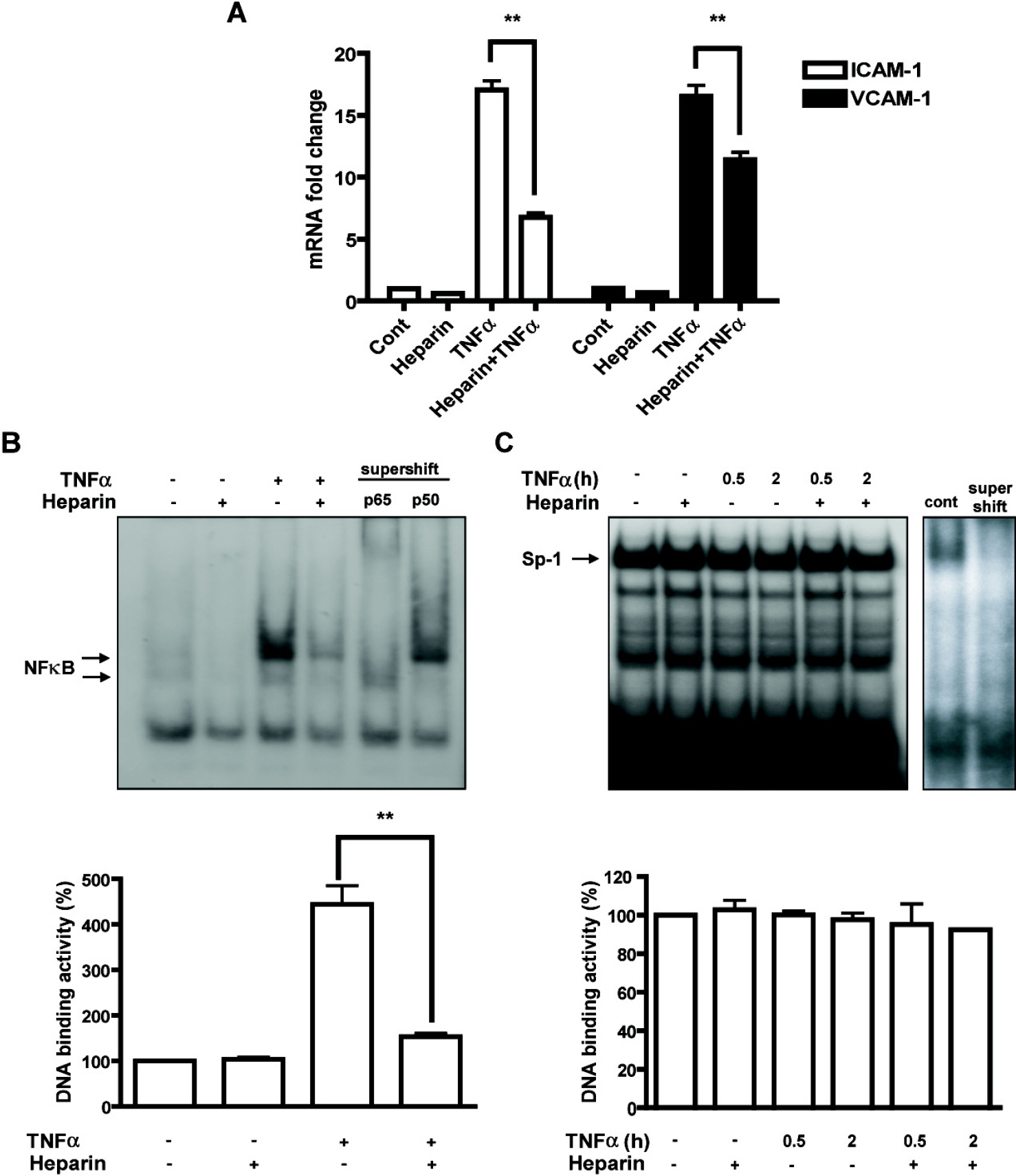
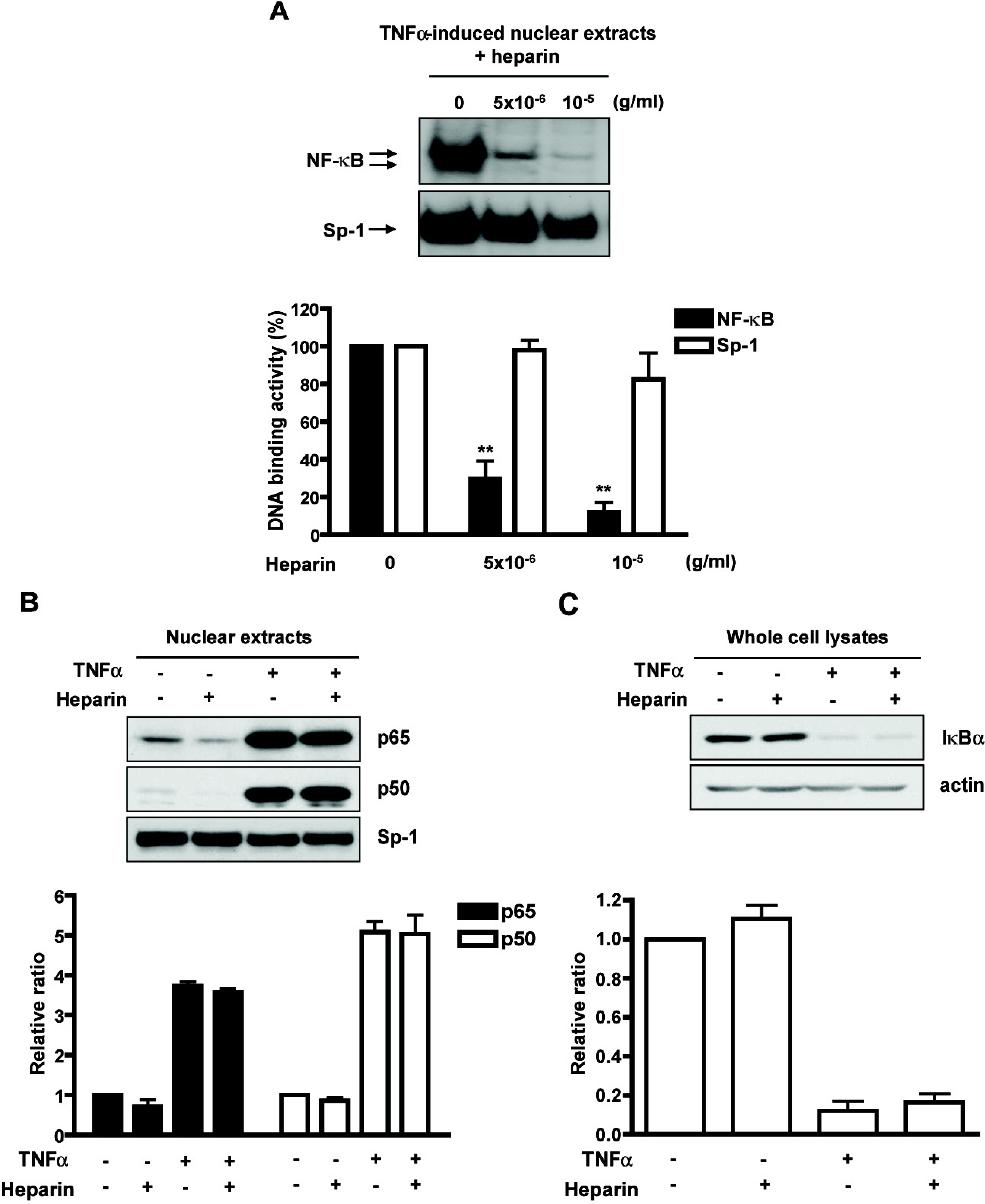
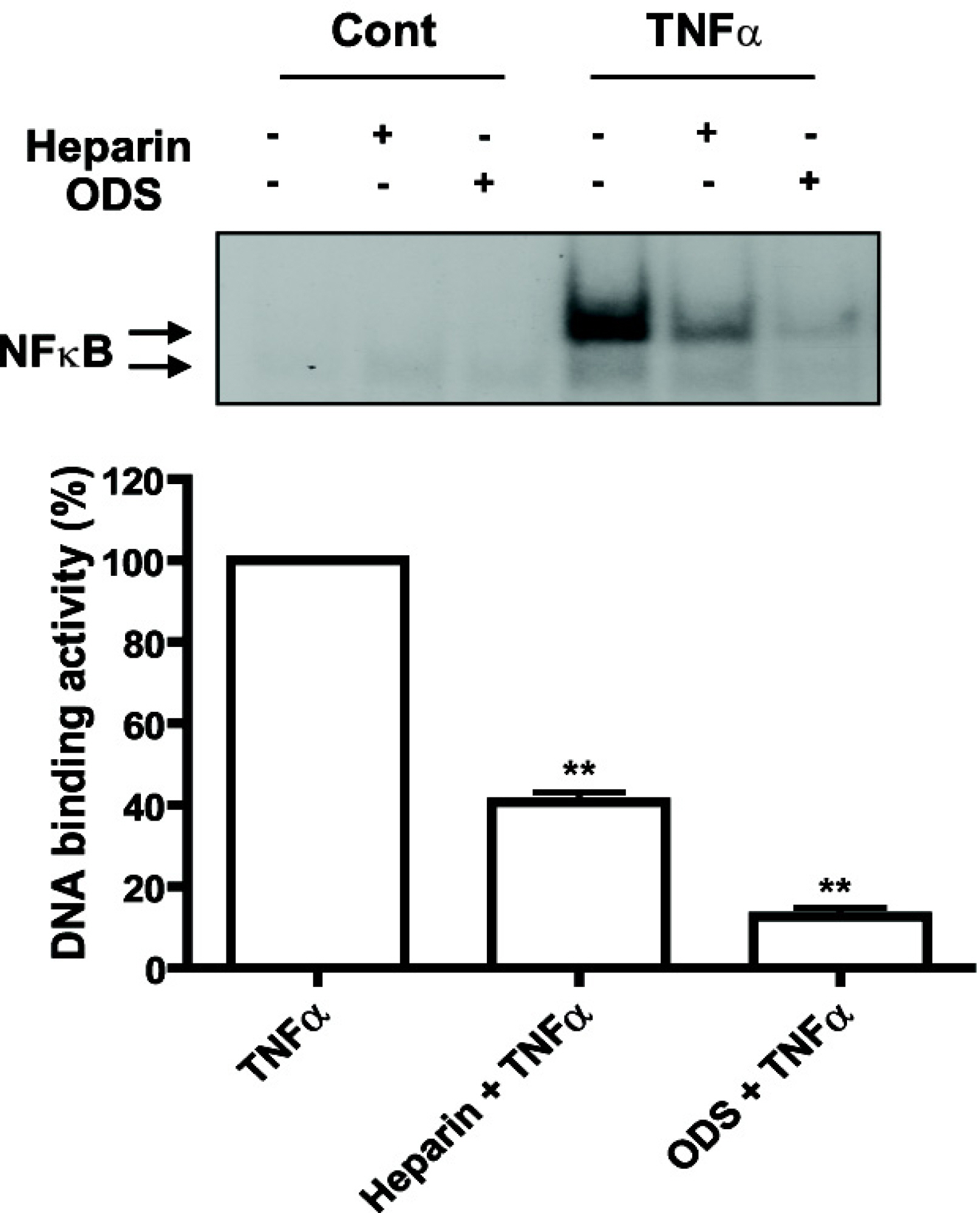
- Heparin is a well-known anticoagulant widely used in various clinical settings. Interestingly, recent studies have indicated that heparin also has anti-inflammatory effects on neuroinflammation-related diseases, such as Alzheimer's disease and meningitis. However, the underlying mechanism of its actions remains unclear. In the present study, we examined the anti-inflammatory mechanism of heparin in cultured cerebral endothelial cells (CECs), and found that heparin inhibited the tumor necrosis factor alpha(TNF alpha)-induced and nuclear factor kappa B (NF-kappa B)-dependent expression of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), which are crucial for inflammatory responses. Heparin selectively interfered with NF-kappa B DNA-binding activity in the nucleus, which is stimulated by TNF alpha. In addition, non-anticoagulant 2,3-O desulfated heparin (ODS) prevented NF-kappa B activation by TNF alpha, suggesting that the anti-inflammatory mechanism of heparin action in CECs lies in heparin's ability to inhibit the expression of cell adhesion molecules, as opposed to its anticoagulant actions.
Keyword
MeSH Terms
-
Alzheimer Disease
Cell Adhesion Molecules
Endothelial Cells
Heparin
Intercellular Adhesion Molecule-1
Meningitis
NF-kappa B
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Cell Adhesion Molecules
Heparin
Intercellular Adhesion Molecule-1
NF-kappa B
Tumor Necrosis Factor-alpha
Vascular Cell Adhesion Molecule-1
Figure
Reference
-
Allan SM., Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2:734–744. 2001.
ArticleAsaba H., Hosoya K., Takanaga H., Ohtsuki S., Tamura E., Takizawa T., Terasaki T. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J Neurochem. 75:1907–1916. 2000.
ArticleBergamaschini L., Donarini C., Rossi E., De Luigi A., Vergani C., De Simoni MG. Heparin attenuates cytotoxic and inflammatory activity of Alzheimer amyloid-beta in vitro. Neurobiol Aging. 23:531–536. 2002.Bergamaschini L., Rossi E., Storini C., Pizzimenti S., Distaso M., Perego C., De Luigi A., Vergani C., De Simoni MG. Peripheral treatment with enoxaparin, a low molecular weight heparin, reduces plaques and beta-amyloid accumulation in a mouse model of Alzheimer's disease. J Neurosci. 24:4181–4186. 2004.Chomczynski P., Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 19:942–945. 1995.Commerford PJ. Heparin after acute myocardial infarction. Cardiovasc Drugs Ther. 11:101–109. 1997.De Vries HE., Kuiper J., de Boer AG., Van Berkel TJ., Breimer DD. The blood-brain barrier in neuroinflammatory diseases. Pharmacol Rev. 49:143–155. 1997.Elsayed E., Becker RC. The impact of heparin compounds on cellular inflammatory responses: a construct for future investigation and pharmaceutical development. J Thromb Thrombolysis. 15:11–18. 2003.Fryer A., Huang YC., Rao G., Jacoby D., Mancilla E., Whorton R., Piantadosi CA., Kennedy T., Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 282:208–219. 1997.Gaffney PR., O'Leary JJ., Doyle CT., Gaffney A., Hogan J., Smew F., Annis P. Response to heparin in patients with ulcerative colitis. Lancet. 337:238–239. 1991.
ArticleGao Y., Li N., Fei R., Chen Z., Zheng S., Zeng X. P-Selectin-mediated acute inflammation can be blocked by chemically modified heparin, RO-heparin. Mol Cells. 19:350–355. 2005.Gloor SM., Wachtel M., Bolliger MF., Ishihara H., Landmann R., Frei K. Molecular and cellular permeability control at the blood-brain barrier. Brain Res Brain Res Rev. 36:258–264. 2001.
ArticleGoedkoop AY., Kraan MC., Picavet DI., de Rie MA., Teunissen MB., Bos JD., Tak PP. Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study. Arthritis Res Ther. 6:R326–334. 2004.Guan H., Hou S., Ricciardi RP. DNA binding of repressor nuclear factor-kappaB p50/p50 depends on phosphorylation of Ser337 by the protein kinase A catalytic subunit. J Biol Chem. 280:9957–9962. 2005.Hou J., Baichwal V., Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 91:11641–11645. 1994.
ArticleJahnke A., Johnson JP. Synergistic activation of intercellular adhesion molecule 1 (ICAM-1) by TNF-alpha and IFN-gamma is mediated by p65/p50 and p65/c-Rel and interferon-responsive factor Stat1 alpha (p91) that can be activated by both IFN-gamma, IFN-alpha. FEBS Lett. 354:220–226. 1994.Jamaluddin M., Wang S., Boldogh I., Tian B., Brasier AR. TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal. 19:1419–1433. 2007.Kavanaugh AF., Schulze-Koops H., Davis LS., Lipsky PE. Repeat treatment of rheumatoid arthritis patients with a murine anti-intercellular adhesion molecule 1 monoclonal antibody. Arthritis Rheum. 40:849–853. 1997.
ArticleLee JH., Lee J., Seo GH., Kim CH., Ahn YS. Heparin inhibits NF-kappaB activation and increases cell death in cerebral endothelial cells after oxygen-glucose deprivation. J Mol Neurosci. 32:145–154. 2007.Levine MN., Raskob G., Beyth RJ., Kearon C., Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 126:287S–310S. 2004.Li Q., Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2:725–734. 2002.Marsh EE 3rd., Adams HP Jr., Biller J., Wasek P., Banwart K., Mitchell V., Woolson R. Use of antithrombotic drugs in the treatment of acute ischemic stroke: a survey of neurologists in practice in the United States. Neurology. 39:1631–1634. 1989.
ArticleMcIntyre KW., Shuster DJ., Gillooly KM., Dambach DM., Pattoli MA., Lu P., Zhou XD., Qiu Y., Zusi FC., Burke JR. A highly selective inhibitor of I kappa B kinase, BMS-345541, blocks both joint inflammation and destruction in collagen-induced arthritis in mice. Arthritis Rheum. 48:2652–2659. 2003.Meng L., Mohan R., Kwok BH., Elofsson M., Sin N., Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 96:10403–10408. 1999.
ArticleMinghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 18:315–321. 2005.
ArticleMrak RE., Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 26:349–354. 2005.
ArticleMuller CW., Rey FA., Sodeoka M., Verdine GL., Harrison SC. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 373:311–317. 1995.Neish AS., Read MA., Thanos D., Pine R., Maniatis T., Collins T. Endothelial interferon regulatory factor 1 cooperates with NF-kappa B as a transcriptional activator of vascular cell adhesion molecule 1. Mol Cell Biol. 15:2558–2569. 1995.
ArticleNode K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin DC., Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 285:1276–1279. 1999.
ArticleOsborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 62:3–6. 1990.
ArticleRead MA., Neish AS., Luscinskas FW., Palombella VJ., Maniatis T., Collins T. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity. 2:493–506. 1995.
ArticleSun H., Dai H., Shaik N., Elmquist WF. Drug efflux transporters in the CNS. Adv Drug Deliv Rev. 55:83–105. 2003.
ArticleTsuji A., Tamai II. Carrier-mediated or specialized transport of drugs across the blood-brain barrier. Adv Drug Deliv Rev. 36:277–290. 1999.
ArticleTurowski P., Adamson P., Greenwood J. Pharmacological targeting of ICAM-1 signaling in brain endothelial cells: potential for treating neuroinflammation. Cell Mol Neurobiol. 25:153–170. 2005.
ArticleUlbrich H., Eriksson EE., Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci. 24:640–647. 2003.
ArticleVermeulen L., De Wilde G., Van Damme P., Vanden Berghe W., Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 22:1313–1324. 2003.
ArticleWang L., Brown JR., Varki A., Esko JD. Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. J Clin Invest. 110:127–136. 2002.
ArticleWeber JR., Angstwurm K., Rosenkranz T., Lindauer U., Freyer D., Burger W., Busch C., Einhaupl KM., Dirnagl U. Heparin inhibits leukocyte rolling in pial vessels and attenuates inflammatory changes in a rat model of experimental bacterial meningitis. J Cereb Blood Flow Metab. 17:1221–1229. 1997.
ArticleZhong H., Voll RE., Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1:661–671. 1998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NADPH Oxidase and Mitochondrial ROS are Involved in the TNF-alpha-induced Vascular Cell Adhesion Molecule-1 and Monocyte Adhesion in Cultured Endothelial Cells
- Effect of cytokines on the expression of cell adhesion molecule and on the adhesion of melanoma cells to endothelial cells
- Effect of Ultraviolet Light on the Expression of Adhesion Molecules and T Lymphocyte Adhesion to Human Dermal Microvascular Endothelial Cells
- Genipin Selectively Inhibits TNF-alpha-activated VCAM-1 But Not ICAM-1 Expression by Upregulation of PPAR-gamma in Human Endothelial Cells
- Resveratrol blunts tumor necrosis factor-alpha-induced monocyte adhesion and transmigration