Nutr Res Pract.
2011 Jun;5(3):192-197.
Effects of Panicum miliaceum L. extract on adipogenic transcription factors and fatty acid accumulation in 3T3-L1 adipocytes
- Affiliations
-
- 1Functional Food & Nutrition Division, National Academy of Agricultural Science, Rural Development Administration, 150 Suin-ro, Gwonseon-gu, Suwon, Gyeonggi 441-707, Korea. dpark@korea.kr
- 2Food Analysis Center, Korea Food Research Institute, Seongnam, Gyeonggi 463-746, Korea.
- 3Department of Food and Nutrition, Sookmyung Women's University, Seoul 140-742, Korea.
- 4Department of Home Economics Education, Kongju National University, Gongju 314-701, Korea.
Abstract
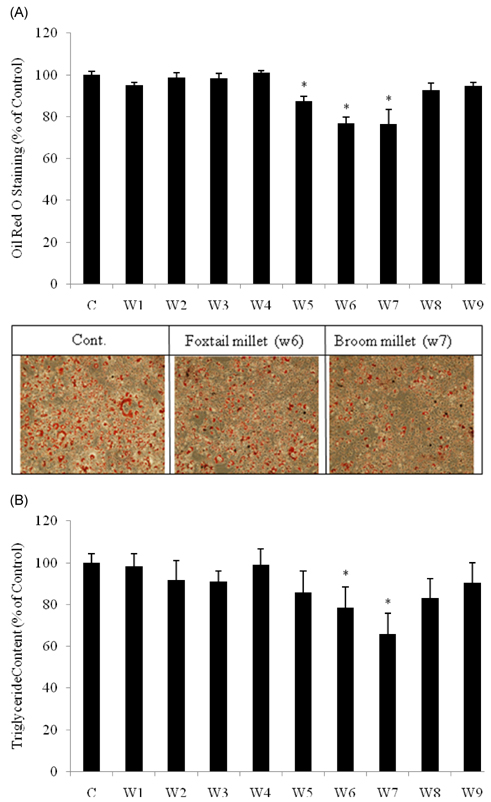
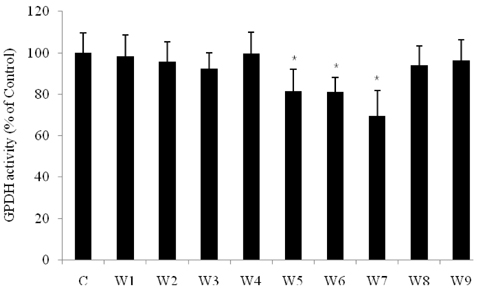
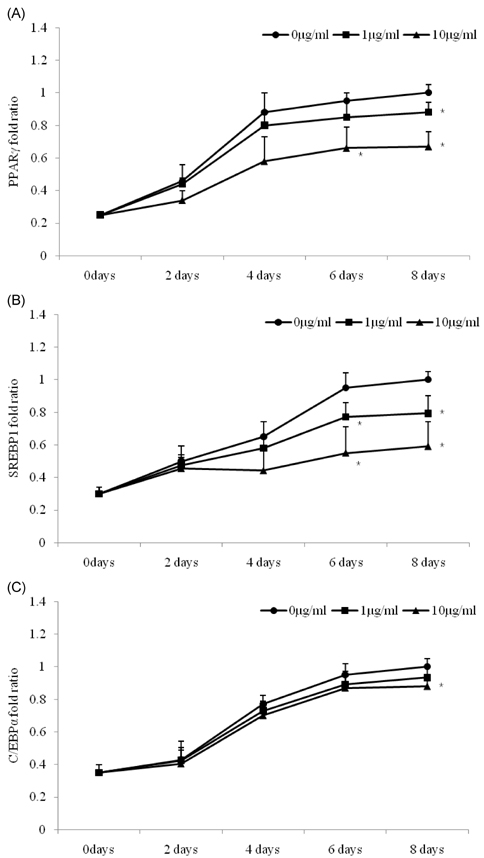
- The dietary intake of whole grains is known to reduce the incidence of chronic diseases such as obesity, diabetes, cardiovascular disease, and cancer. To investigate whether there are anti-adipogenic activities in various Korean cereals, we assessed water extracts of nine cereals. The results showed that treatment of 3T3-L1 adipocytes with Sorghum bicolor L. Moench, Setaria italica Beauvois, or Panicum miliaceum L. extract significantly inhibited adipocyte differentiation, as determined by measuring oil red-O staining, triglyceride accumulation, and glycerol 3-phosphate dehydrogenase activity. Among the nine cereals, P. miliaceum L. showed the highest anti-adipogenic activity. The effects of P. miliaceum L. on mRNA expression of peroxisome proliferator-activated receptor-gamma, sterol regulatory element-binding protein 1, and the CCAAT/enhancer binding protein-alpha were evaluated, revealing that the extract significantly decreased the expression of these genes in a dose-dependent manner. Moreover, P. miliaceum L. extract changed the ratio of monounsaturated fatty acids to saturated fatty acids in adipocytes, which is related to biological activity and cell characteristics. These results suggest that some cereals efficiently suppress adipogenesis in 3T3-L1 adipocytes. In particular, the effect of P. miliaceum L. on adipocyte differentiation is associated with the downregulation of adipogenic genes and fatty acid accumulation in adipocytes.
Keyword
MeSH Terms
-
3T3-L1 Cells
Adipocytes
Adipogenesis
Cardiovascular Diseases
Edible Grain
Chronic Disease
Down-Regulation
Fatty Acids
Fatty Acids, Monounsaturated
Glycerol
Glycerophosphates
Incidence
Obesity
Oxidoreductases
Panicum
Peroxisomes
RNA, Messenger
Setaria Plant
Sorghum
Sterol Regulatory Element Binding Protein 1
Transcription Factors
Water
Fatty Acids
Fatty Acids, Monounsaturated
Glycerol
Glycerophosphates
Oxidoreductases
RNA, Messenger
Sterol Regulatory Element Binding Protein 1
Transcription Factors
Water
Figure
Reference
-
1. Barsh GS, Farooqi IS, O'Rahilly S. Genetics of body-weight regulation. Nature. 2000. 404:644–651.
Article2. Dugee O, Khor GL, Lye MS, Luvsannyam L, Janchiv O, Jamyan B, Esa N. Association of major dietary patterns with obesity risk among Mongolian men and women. Asia Pac J Clin Nutr. 2009. 18:433–440.3. Misra A, Rastogi K, Joshi SR. Whole grains and health: perspective for Asian Indians. J Assoc Physicians India. 2009. 57:155–162.4. Williams PG, Grafenauer SJ, O'Shea JE. Cereal grains, legumes, and weight management: a comprehensive review of the scientific evidence. Nutr Rev. 2008. 66:171–182.
Article5. Cho EJ, Rahman MA, Kim SW, Baek YM, Hwang HJ, Oh JY, Hwang HS, Lee SH, Yun JW. Chitosan oligosaccharides inhibit adipogenesis in 3T3-L1 adipocytes. J Microbiol Biotechnol. 2008. 18:80–87.6. Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006. 7:885–896.
Article7. van Dijk SJ, Feskens EJ, Bos MB, Hoelen DW, Heijligenberg R, Bromhaar MG, de Groot LC, de Vries JH, Müller M, Afman LA. A saturated fatty acid-rich diet induces an obesity-linked proinflammatory gene expression profile in adipose tissue of subjects at risk of metabolic syndrome. Am J Clin Nutr. 2009. 90:1656–1664.
Article8. Phillips CM, Goumidi L, Bertrais S, Field MR, Peloso GM, Shen J, McManus R, Hercberg S, Lairon D, Planells R, Roche HM. Dietary saturated fat modulates the association between STAT3 polymorphisms and abdominal obesity in adults. J Nutr. 2009. 139:2011–2017.
Article9. Bos MB, de Vries JH, Feskens EJ, van Dijk SJ, Hoelen DW, Siebelink E, Heijligenberg R, de Groot LC. Effect of a high monounsaturated fatty acids diet and a Mediterranean diet on serum lipids and insulin sensitivity in adults with mild abdominal obesity. Nutr Metab Cardiovasc Dis. 2010. 20:591–598.
Article10. Lai CQ, Parnell LD, Arnett DK, García-Bailo B, Tsai MY, Kabagambe EK, Straka RJ, Province MA, An P, Borecki IB, Tucker KL, Ordovás JM. WDTC1, the ortholog of Drosophila adipose gene, associates with human obesity, modulated by MUFA intake. Obesity (Silver Spring). 2009. 17:593–600.
Article11. Choi BH, Kim YH, Ahn IS, Ha JH, Byun JM, Do MS. The inhibition of inflammatory molecule expression on 3T3-L1 adipocytes by berberine is not mediated by leptin signaling. Nutr Res Pract. 2009. 3:84–88.
Article12. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001. 25:402–408.
Article13. He ML, Hnin TM, Kuwayama H, Mir PS, Okine EK, Hidari H. Effect of conjugated linoleic acid type, treatment period, and dosage on differentiation of 3T3 cells. Lipids. 2006. 41:937–949.
Article14. Kramer JK, Fellner V, Dugan ME, Sauer FD, Mossoba MM, Yurawecz MP. Evaluating acid and base catalysts in the methylation of milk and rumen fatty acids with special emphasis on conjugated dienes and total trans fatty acids. Lipids. 1997. 32:1219–1228.
Article15. Kozak LP, Jensen JT. Genetic and developmental control of multiple forms of L-glycerol 3-phosphate dehydrogenase. J Biol Chem. 1974. 249:7775–7781.
Article16. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998. 78:783–809.
Article17. Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000. 16:145–171.
Article18. Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000. 289:950–953.
Article19. Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR. Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol. 2004. 24:1918–1929.
Article20. Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005. 1:27–39.
Article21. Kim JB, Wright HM, Wright M, Spiegelman BM. ADD1/SREBP1 activates PPARγ through the production of endogenous ligand. Proc Natl Acad Sci U S A. 1998. 95:4333–4337.
Article22. Siener R, Alteheld B, Terjung B, Junghans B, Bitterlich N, Stehle P, Metzner C. Change in the fatty acid pattern of erythrocyte membrane phospholipids after oral supplementation of specific fatty acids in patients with gastrointestinal diseases. Eur J Clin Nutr. 2010. 64:410–418.
Article23. Koenitzer JR, Freeman BA. Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010. 1203:45–52.
Article24. Søgaard R, Werge TM, Bertelsen C, Lundbye C, Madsen KL, Nielsen CH, Lundbaek JA. GABAA receptor function is regulated by lipid bilayer elasticity. Biochemistry. 2006. 45:13118–13129.
Article25. Collins JM, Neville MJ, Hoppa MB, Frayn KN. De novo lipogenesis and stearoyl-CoA desaturase are coordinately regulated in the human adipocyte and protect against palmitate-induced cell injury. J Biol Chem. 2010. 285:6044–6052.
Article26. Scaglia N, Igal RA. Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol. 2008. 33:839–850.
Article27. Jaureguiberry MS, Tricerri MA, Sanchez SA, Garda HA, Finarelli GS, Gonzalez MC, Rimoldi OJ. Membrane organization and regulation of cellular cholesterol homeostasis. J Membr Biol. 2010. 234:183–194.
Article28. He ML, Wang Y, You JS, Mir PS, McAllister TA. Effect of a seaweed extract on fatty acid accumulation and glycerol-3-phosphate dehydrogenase activity in 3T3-L1 adipocytes. Lipids. 2009. 44:125–132.
Article29. Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010. 59:386–396.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The micosporine-like amino acids-rich aqueous methanol extract of laver (Porphyra yezoensis) inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Carnosic Acid Inhibits Lipid Accumulation in 3T3-L1 Adipocytes Through Attenuation of Fatty Acid Desaturation
- Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte
- Anti-adipogenic Effect and Mechanism in 3T3-L1 Preadipocyte Differentiation by Salvianolic Acid B
- Anti-adipogenic effect of mulberry leaf ethanol extract in 3T3-L1 adipocytes