Lab Med Online.
2014 Jan;4(1):22-27.
Evaluation of the T-KB-H and 3-HB Kits for the Measurement of Serum Ketone and beta-Hydroxybutyric Acid
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea. songjhcp@snu.ac.kr
- 2Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea.
- 3Department of Laboratory Medicine, Seoul National University Hospital, Korea.
Abstract
- BACKGROUND
Diabetes mellitus and alcohol consumption are the most common causes of ketoacidosis in adults. Recently, beta-hydroxybutyric acid (betaHBA) was reported to be a potential serum biomarker in the diagnosis and monitoring of ketoacidosis. We evaluated the performance of T-KB-H and 3-HB kits for the measurement of ketone bodies [acetoacetate (AcAc)+betaHBA] and betaHBA, respectively.
METHODS
Quantitative enzymatic assays were performed using the T-KB-H and 3-HB kits (Nittobo Medical Co., Japan) and the Architect ci16200 Integrated System (Abbott Laboratories, USA). Simultaneously, the ketone body levels in these serum samples were determined by gas chromatography-mas spectrometry (GC-MS). We evaluated precision and linearity of these kits and correlation with GC-MS, and established reference intervals in children and adults.
RESULTS
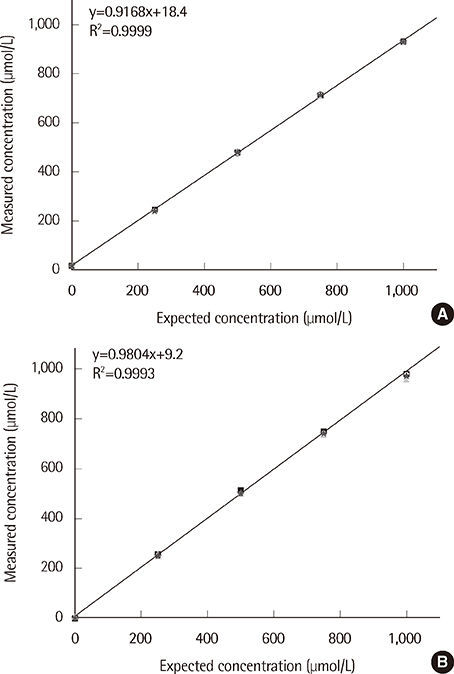
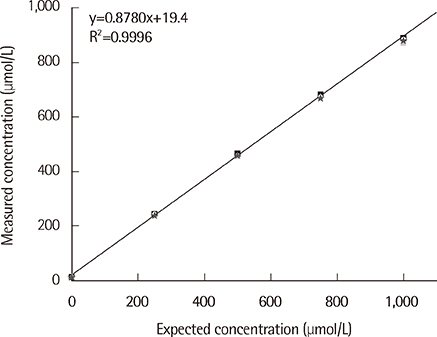
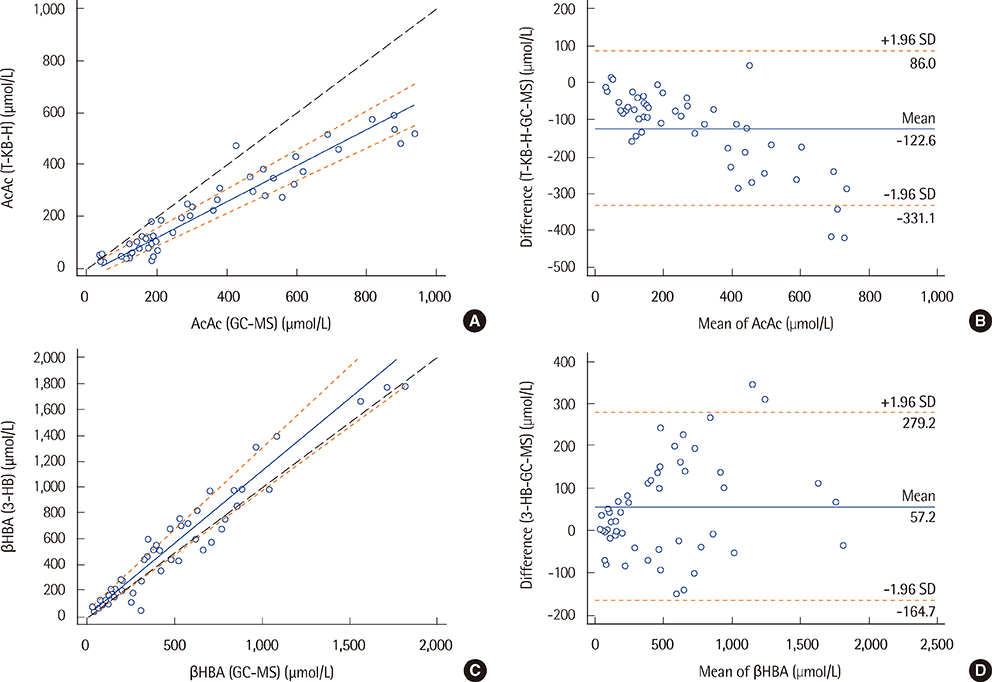
The coefficients of variation for the T-KB-H and 3-HB kits were less than 4.0% at analyte levels of 50, 100, and 400 micromol/L. Linearity was observed for AcAc and betaHBA over a 0-1,000 micromol/L range (R2<0.99). Results from the T-KB-H and 3-HB kits were in good agreement with those from the GC-MS analysis, with correlation coefficients of 0.94 for AcAc and 0.96 for betaHBA. Reference intervals determined for the T-KB-H kit were 9.8-270.1 micromol/L and 18.5-531.8 micromol/L in children and adults, respectively. For the 3-HB kit, the reference intervals were 6.4-234.0 micromol/L and 16.0-437.2 micromol/L in children and adults, respectively.
CONCLUSIONS
The T-KB-H and 3-HB kits displayed good precision, clinically acceptable linearity, and reliable correlation with an established assay. This indicates that the kits can be used clinically for measuring serum ketone bodies.
Keyword
MeSH Terms
Figure
Reference
-
1. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999; 15:412–426.
Article2. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011; 34:e61–e99.
Article3. Helander A, Beck O, Jones AW. Laboratory testing for recent alcohol consumption: comparison of ethanol, methanol, and 5-hydroxytryptophol. Clin Chem. 1996; 42:618–624.
Article4. Hassan HM, Cooper GA. Determination of β-hydroxybutyrate in blood and urine using gas chromatography-mass spectrometry. J Anal Toxicol. 2009; 33:502–507.
Article5. Marliss EB, Ohman JL Jr, Aoki TT, Kozak GP. Altered redox state obscuring ketoacidosis in diabetic patients with lactic acidosis. N Engl J Med. 1970; 283:978–980.
Article6. Foreback CC. β-Hydroxybutyrate and acetoacetate levels. Am J Clin Pathol. 1997; 108:602–604.
Article7. Clinical and Laboratory Standards Institute. EP05-A2. Evaluation of precision performance of quantitative measurement methods; approved guideline. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2004.8. Clinical and Laboratory Standards Institute. Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. EP6-A. Wayne, PA: Clinical and Laboratory Standards Institute;2003.9. Clinical and Laboratory Standards Institute. Method comparison and bias estimation using patient samples; Approved Guideline. EP9-2. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2002.10. Sheikh-Ali M, Karon BS, Basu A, Kudva YC, Muller LA, Xu J, et al. Can serum β-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008; 31:643–647.
Article11. Yu HY, Agus M, Kellogg MD. Clinical utility of Abbott Precision Xceed Pro® ketone meter in diabetic patients. Pediatr Diabetes. 2011; 12:649–655.
Article12. Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. St.Louis: Saunders/Elsevier;2012. p. 1440–1441.13. Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, et al. Medical aspects of ketone body metabolism. Clin Invest Med. 1995; 18:193–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the Autokit Total Ketone Bodies
- Accuracy of capillary blood 3-beta-hydroxybutyrate determination for the detection and treatment of canine diabetic ketoacidosis
- Effect of beta-Hydroxybutyrate on Flurothyl-Induced Seizure Susceptibility
- A Study on the Age-Dependent Ketosis Induced by the Ketogenic Diet
- Effects of Anti-thyroglobulin Antibody on the Measurement of Thyroglobulin: Differences Between Immunoradiometric Assay Kits Available