Lab Anim Res.
2015 Sep;31(3):125-133. 10.5625/lar.2015.31.3.125.
Toxicity of antioxidative extract collected from Styela clava tunics in ICR mice
- Affiliations
-
- 1Department of Biomaterials Science, College of Natural Resources and Life Science/Life and Industry Convergence Research Institute, Pusan National University, Miryang, Korea. dyhwang@pusan.ac.kr
- 2Department of Life Science and Environmental Biochemistry, College of Natural Resources & Life Science, Pusan National University, Miryang, Korea.
- 3Department of Clinical Laboratory Science, College of Nursing and Healthcare Science, Dong-Eui University, Busan, Korea.
- KMID: 2312129
- DOI: http://doi.org/10.5625/lar.2015.31.3.125
Abstract
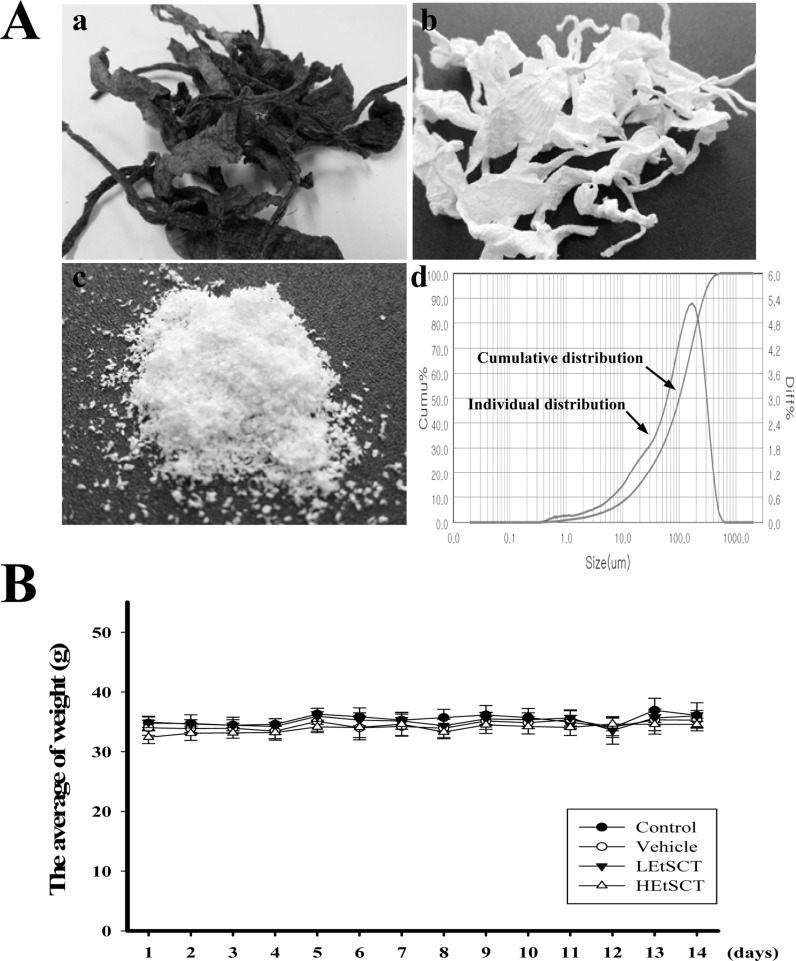
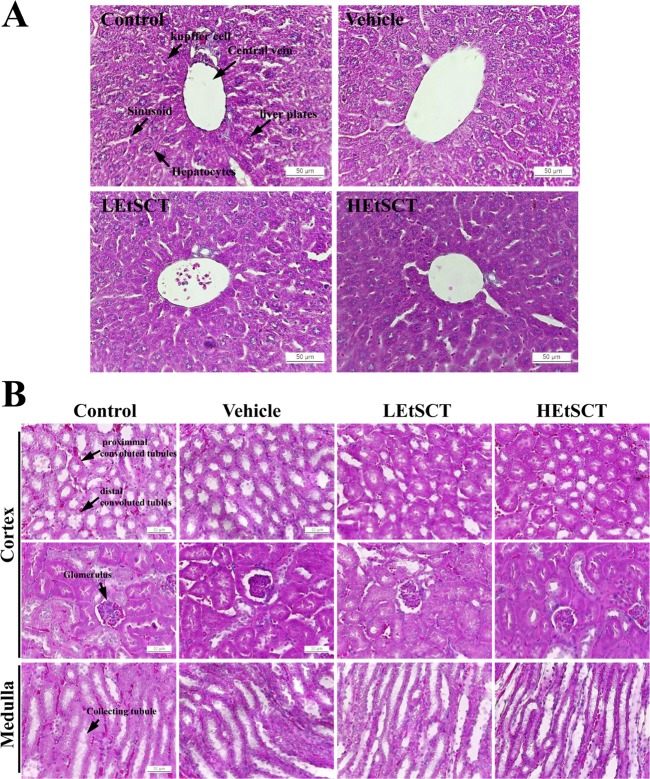
- Some polymers and bioactive compounds derived from Styela clava tunic (SCT) have been reported as traditional medicine for the treatment of inflammation, oxidative stress and surgical wounds although there is little scientific evidence of their liver and kidney toxicity. To investigate the toxicity of ethanol extracts of SCT (EtSCT) in the liver and kidney of ICR mice, alterations in related markers including body weight, organ weight, urine composition, liver pathology and kidney pathology were analyzed following oral administration of 50 and 100 mg/kg body weight/day of EtSCT for 14 days. EtSCT showed a high level of free radical scavenging activity for DPPH (93.1%) and NO (16.2%) as well as the presence of 14.8 mg/mL of flavonoids and 36.2 mg/mL of phenolics, while EtSCT treated groups did not show any significant alterations in the body and organ weight, clinical phenotypes, urine parameters or mice mortality when compared with the vehicle treated group. In addition, constant levels of serum biochemical markers including alanine phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN) and serum creatinine (CRE) were maintained. Moreover, no specific histopathological features induced by most toxic compounds were observed in liver and kidney sections stained with hematoxilin and eosin. Therefore, the present results indicate that EtSCT with strong antioxidant activity cannot induce any specific toxicity in liver and kidney organs of ICR at doses of 100 mg/kg body weight/day.
MeSH Terms
-
Administration, Oral
Alanine
Alanine Transaminase
Animals
Aspartate Aminotransferases
Biomarkers
Blood Urea Nitrogen
Body Weight
Creatinine
Eosine Yellowish-(YS)
Ethanol
Flavonoids
Inflammation
Kidney
Liver
Medicine, Traditional
Mice
Mice, Inbred ICR*
Mortality
Organ Size
Oxidative Stress
Pathology
Phenol
Phenotype
Polymers
Wounds and Injuries
Alanine
Alanine Transaminase
Aspartate Aminotransferases
Creatinine
Eosine Yellowish-(YS)
Ethanol
Flavonoids
Phenol
Polymers
Figure
Reference
-
1. Richard CK, Walter YR. Market potential for Styela clava, a non-indigenous pest invading New England coastal waters. Aquat Invasions. 2009; 4(1):295–297.2. Lee SM, Kang EJ, Go TH, Jeong SY, Park GT, Lee HS, Hwang DY, Jung YJ, Son HJ. Screening of biological activity of solvent extract from Styela clava tunic for fishery waste recycling. J Environ Sci Int. 2014; 23(1):89–96.3. Song SH, Kim JE, Lee YJ, Kwak MH, Sung GY, Kwon SH, Son HJ, Lee HS, Jung YJ, Hwang DY. Cellulose film regenerated from Styela clava tunics have biodegradability, toxicity and biocompatibility in the skin of SD rats. J Mater Sci Mater Med. 2014; 25(6):1519–1530. PMID: 24577945.4. Jung YJ. Properties of regenerated cellulose films prepared from the tunicate Styela clava. J Korean Fish Soc. 2008; 41(4):237–242.5. Seong KY, Koh EK, Lee SH, Kwak MH, Son HJ, Lee HS, Hwang DY, Jung YJ. Preparation and characterization of high absorptive cellulose film derived from Styela clava tunic for wound dressing. Tex Color Fin. 2015; 27(1):70–79.6. Kwak MH, Go J, Kim JE, Lee YJ, Lee SH, Lee HS, Son HJ, Jung YJ, Hwang DY. Property and efficacy analysis of hydrocolloid membrane containing Styela clava tunic on the wound repair of skin in SD rats. Biomater Res. 2013; 17(3):91–101.7. Kim SM, Lee JH, Cho JA, Lee SC, Lee SK. Development of a bioactive cellulose membrane from sea squirt skin for bone regeneration-A preliminary research. J Korean Assoc Oral Maxillofac Surg. 2005; 31:440–453.8. Ahn SH, Jung SH, Kang SJ, Jeong TS, Choi BD. Extraction of glycosaminoglycans from Styela clava tunic. Korean J Biotechnol Bioeng. 2003; 18(3):180–185.9. Xu CX, Jin H, Chung YS, Shin JY, Woo MA, Lee KH, Palmos GN, Choi BD, Cho MH. Chondroitin sulfate extracted from the Styela clava tunic suppresses TNF-alpha-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-kappaB signal in JB6 cells. Cancer Lett. 2008; 264(1):93–100. PMID: 18295395.10. Loda MN, Kang SJ, Choi BD. Antioxidative activity of carotenoids in Mideodeok Styela clava. Fish Aquat Sci. 2011; 14(4):243–249.11. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16(3):144–158.12. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999; 64:555–559.
Article13. Oh H, Ko EK, Kim DH, Jang KK, Park SE, Lee HS, Kim YC. Secoiridoid glucosides with free radical scavenging activity from the leaves of Syringa dilatata. Phytother Res. 2003; 17(4):417–419. PMID: 12722154.14. Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994; 201(2):748–755. PMID: 8003011.15. Millar H. The identity of the ascidians Styela mammiculata Carlisle and Styela clava Herdman. J Mar Biol Assoc UK. 1960; 39:509–511.16. Carlisle DB. Styela mammiculata n. sp. a new species of ascidian from the Plymouth area. J Mar Biol Assoc UK. 1954; 33:329–334.17. Kang PA, Kim Y, Yoon DS. Studies on the hanging culture of oyster, Crassostrea gigas, in the Korean coastal waters 4 On the fouling organisms associated with culturing oysters at the oyster culture farms in Chungmu. Bull Natl Fish Res Dev Agency (Korea). 1980; 25:29–34.18. Davis MH, Davis ME. First record of Styela clava (Tunicata: Ascidiacea) from the Mediterranean Sea. Aquat Invasions. 2008; 3:125–132.19. Park JH, Suh YC. GIS-Based suitable site selection for aquaculture using scope for growth of Styela clava. J Korean Assoc Geogr Inf Stud. 2013; 16(3):81–90.20. Shin YK, Park JJ, Park MS, Myeong JI, Hur YB. Effect of temperature and dissolved oxygen on the survival rate and physiological response of the warty sea squirt Styela clava. Korean J Environ Biol. 2014; 32(3):216–224.21. Ahn SH, Jung SH, Kang SJ, Jeong TS, Choi BD. Extraction of glycosaminoglycans from Styela clava tunic. Korean J Biotechnol Bioeng. 2003; 18:180–185.22. Bissell DM, Gores GJ, Laskin DL, Hoofnagle JH. Drug-induced liver injury: mechanisms and test systems. Hepatology. 2001; 33(4):1009–1013. PMID: 11283870.
Article23. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007; 35(5):742–750. PMID: 17849357.
Article24. Kim JJ, Kim SJ, Kim SH, Park HR, Lee SC. Antioxidant and anticancer activities of extracts from Styla plicata. J Korean Soc Food Sci Nutr. 2005; 34:937–941.25. Kim JJ, Kim SJ, Kim SH, Park HR, Lee SC. Antioxidant and anticancer activities of extracts from Styela clava according to the processing methods and solvents. J Korean Soc Food Sci Nutr. 2006; 35:278–283.26. Choi SY, Kim YC. Alleviative effects of jujube water extract on the inflammation and barrier damage in hairless mice skin. Korean J Environ Toxicol. 2009; 24(4):351–357.27. Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, Berens W, Beer JZ, Hearing VJ. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006; 20(9):1486–1488. PMID: 16793869.
Article28. Erickson KL, McNeill CJ, Gershwin ME, Ossmann JB. Influence of dietary fat concentration and saturation on immune ontogeny in mice. J Nutr. 1980; 110(8):1555–1572. PMID: 7400846.
Article29. Cho MS, Kim WY. Effects of dietary fat level on the aging process of the fibroblast cells and immune function in rats of different ages. Korean J Nutr. 1991; 24:431–441.30. Cho YJ. Effects of dietary supplement of mayonnaise on lipid metabolism in rats. J Korean Soc Food Sci Nutr. 1999; 28(4):895–900.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Acute and Sub-acute Oral Toxicity Effect of Aquilaria malaccensis Leaves Aqueous Extract in Male ICR Mice
- An Experimental Study on the Protective Effects of Ginseng Extract to Oxygen Toxicity
- Anti-inflammatory effect of enzymatic hydrolysates from Styela clava flesh tissue in lipopolysaccharide-stimulated RAW 264.7 macrophages and in vivo zebrafish model
- Effect of Maengjong-Juk ( Phyllostachys Pubescens) Extract Coated Rice Diet on Antioxidative System of C57BL/6 Mice Fed Atherogenic Diet
- Immunogenicity of a Combined Hepatitis A and B Vaccine in ICR Mice