Lab Anim Res.
2012 Mar;28(1):1-9. 10.5625/lar.2012.28.1.1.
Differential expression of caveolins and myosin heavy chains in response to forced exercise in rats
- Affiliations
-
- 1Cardiovascular & Metabolic Disease Center, College of Biomedical Science & Engineering, Inje University, Gimhae, Korea.
- 2Department of Rehabilitation Science in Interdisciplinary PhD Program, Inje University, Gimhae, Korea.
- 3National Primate Research Center, Korea Research Institute of Bioscience and Biotechnology, Ochang, Korea. yonghong@inje.ac.kr
- KMID: 2312086
- DOI: http://doi.org/10.5625/lar.2012.28.1.1
Abstract
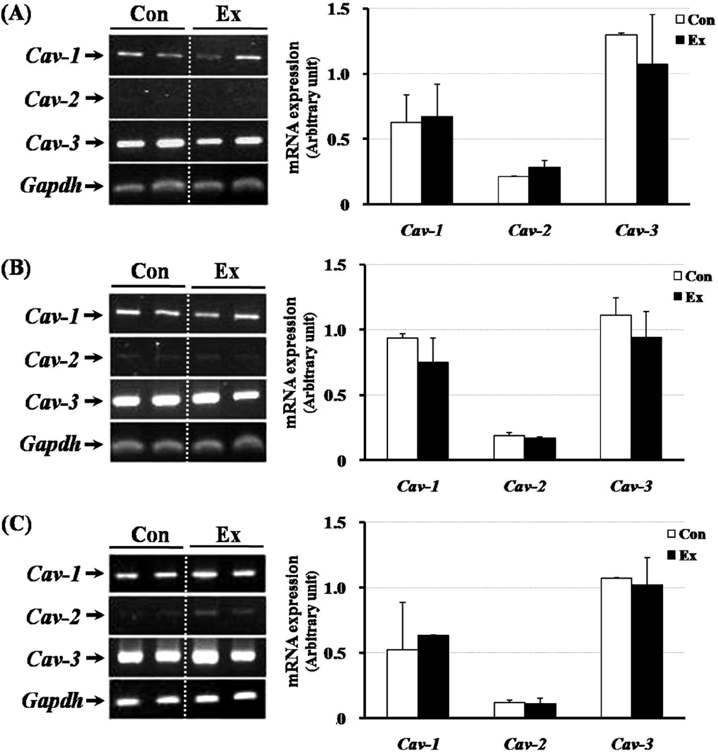
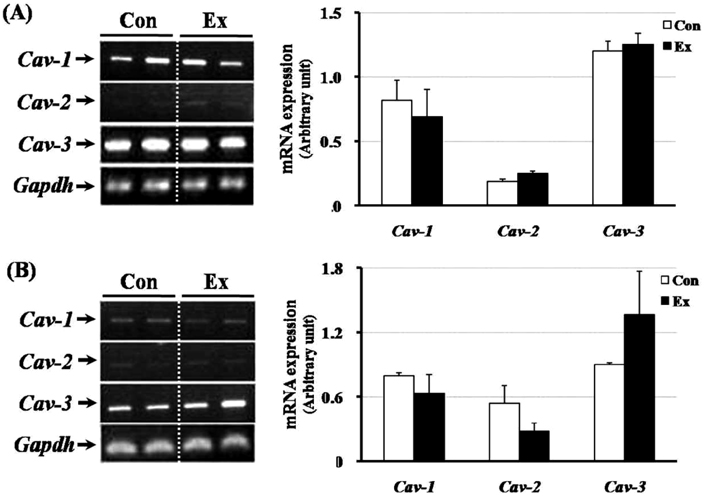
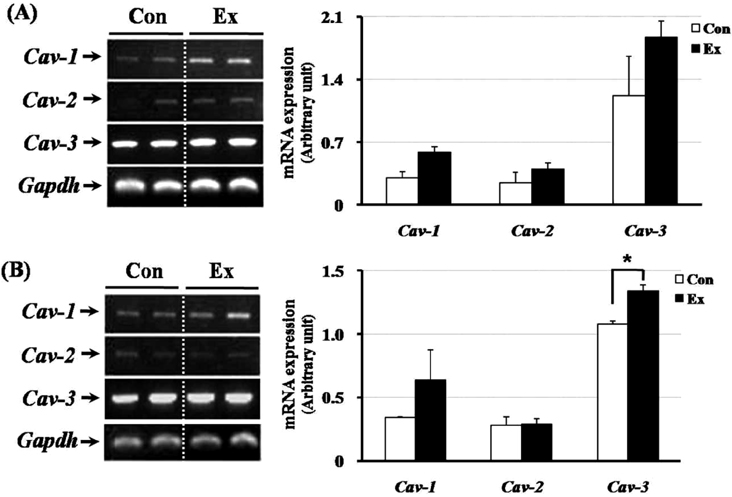
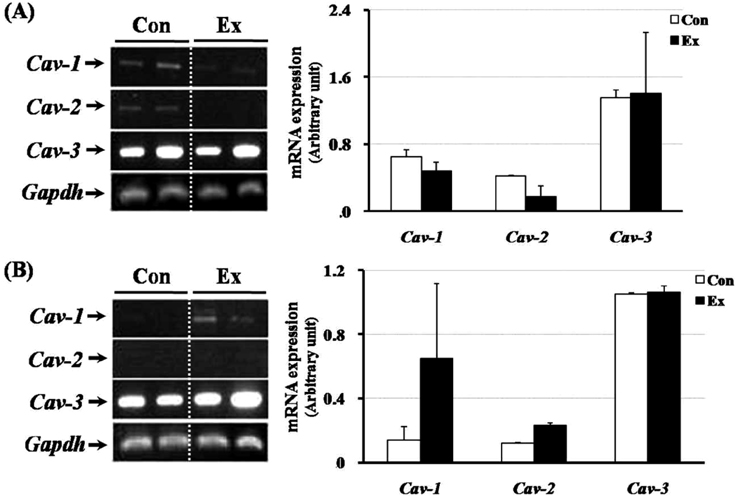
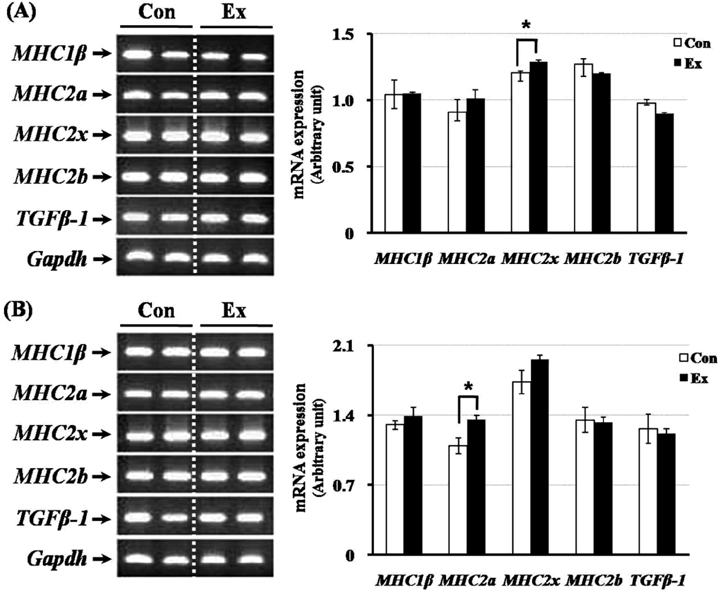
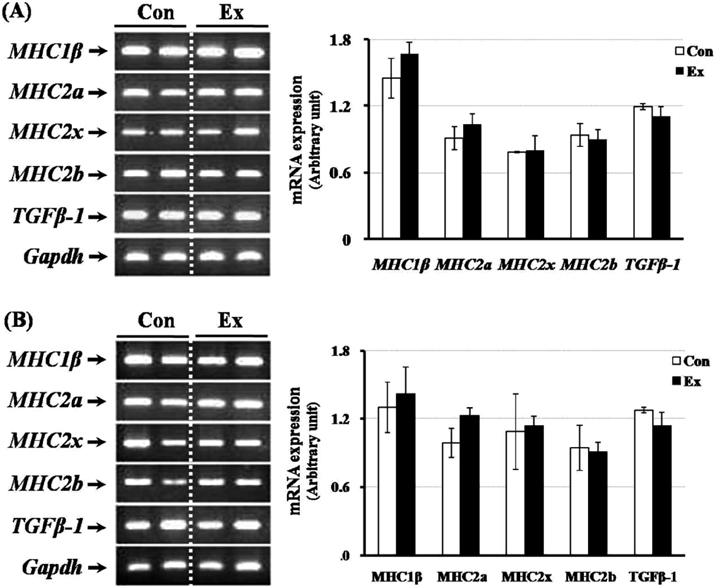
- Exercise training can improve strength and lead to adaptations in the skeletal muscle and nervous systems. Skeletal muscles can develop into two types: fast and slow, depending on the expression pattern of myosin heavy chain (MHC) isoforms. Previous studies reported that exercise altered the distribution of muscle fiber types. It is not currently known what changes in the expression of caveolins and types of muscle fiber occur in response to the intensity of exercise. This study determined the changes in expression of caveolins and MHC type after forced exercise in muscular and non-muscular tissues in rats. A control (Con) group to which forced exercise was not applied and an exercise (Ex) group to which forced exercise was applied. Forced exercise, using a treadmill, was introduced at a speed of 25 m/min for 30 min, 3 times/day (07:00, 15:00, 23:00). Homogenized tissues were applied to extract of total RNA for further gene analysis. The expression of caveolin-3 and MHC2a in the gastrocnemius muscle of female rats significantly increased in the Ex group compared with the Con group (P<0.05). Furthermore, in the gastrocnemius muscle of male rats, the expression of MHC2x was significantly different between the two groups (P<0.05). There was an increased expression in caveolin-3 and a slightly decreased expression in TGFbeta-1 in muscular tissues implicating caveolin-3 influences the expression of MHC isoforms and TGFbeta-1 expression. Eventually, it implicates that caveolin-3 has positive regulatory function in muscle atrophy induced by neural dysfunction with spinal cord injury or stroke.
Keyword
MeSH Terms
Figure
Reference
-
1. Hood DA, Saleem A. Exercise-induced mitochondrial biogenesis in skeletal muscle. Nutr Metab Cardiovasc Dis. 2007. 17(5):332–337.2. Sharma HS, Cervós-Navarro J, Dey PK. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neuroscience Research. 1991. 10(3):211–221.3. Medeiros A, Vanzelli AS, Rosa KT, Irigoyen MC, Brum PC. Effect of exercise training and carvedilol treatment on cardiac function and structure in mice with sympathetic hyperactivity-induced heart failure. Braz J Med Biol Res. 2008. 41(9):812–817.4. Kovanen V, Suominen H. Effects of age and life-time physical training on fiber composition of slow and fast skeletal muscle in rats. Pflugers Arch. 1987. 408(6):543–551.5. Hammeren J, Powers S, Lawler J, Criswell D, Martin D, Lowenthal D, Pollock M. Exercise training-induced alterations in skeletal muscle oxidative and antioxidant enzyme activity in senescent rats. Int J Sports Med. 1992. 13(5):412–416.6. Moran M, Delgado J, Gonzalez B, Manso R, Megías A. Responses of rat myocardial antioxidant defences and heat shock protein HSP72 induced by 12 and 24 week treadmill training. Acta Physiol Scand. 2004. 180(2):157–166.7. Narici MV, Reeves ND, Morse CI, Maganaris CN. Muscular adaptations to resistance exercise in the elderly. J Musculoskelet Neuronal Interact. 2004. 4(2):161–164.8. Silbermann M, Finkelbrand S, Weiss A, Gershon D, Reznick A. Morphometric analysis of aging skeletal muscle following endurance training. Muscle Nerve. 1983. 6(2):136–142.9. Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985. 403(4):369–376.10. Wernig A, Irintchev A, Weisshaupt P. Muscle injury, cross-sectional area, and fiber type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990. 428:639–652.11. Irintchev A, Wernig A. Muscle damage and repair in voluntary running mice: Strain and muscle differences. Cell Tissue Res. 1987. 249(3):509–521.12. Pette D. Training effects on the contractile apparatus. Acta Physiol Scand. 1998. 162(3):367–376.13. Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001. 276(47):43524–43533.14. Talmadge RJ, Roy RR, Edgerton VR. Muscle fiber types and function. Curr Opin Rheumatol. 1993. 5(6):695–705.15. Braith RW, Magyari PM, Pierce GL, Edwards DG, Hill JA, White LJ, Aranda JM Jr. Effects of resistance exercise on skeletal muscle myopathy in heart transplant recipients. Am J Cardiol. 2005. 95(10):1192–1198.16. Oh YS, Kim HJ, Ryu SJ, Cho KA, Park YS, Park H, Kim M, Kim CK, Park SC. Exercise type and muscle fiber specific induction of caveolin-1 expression for insulin sensitivity of skeletal muscle. Exp Mol Med. 2007. 39(3):395–401.17. Lisanti MP, Scherer PE, Tang Z, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: A signalling hypothesis. Trends Cell Biol. 1994. 4(7):231–235.18. Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997. 272(48):30429–30438.19. Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: Implications for signaling and cellular actions. Mol Endocrinol. 2002. 16(1):100–115.20. Capanni C, Sabatelli P, Mattioli E, Ognibene A, Columbaro M, Lattanzi G, Merlini L, Minetti C, Maraldi NM, Squarzoni S. Dysferlin in a hyperCKaemic patient with caveolin 3 mutation and in C2C12 cells after p38 MAP kinase inhibition. Exp Mol Med. 2003. 35(6):538–544.21. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997. 387(6628):83–90.22. Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002. 296(5572):1486–1488.23. Reisz-Porszasz S, Bhasin S, Artaza JN, Shen R, Sinha-Hikim I, Hogue A, Fielder TJ, Gonzalez-Cadavid NF. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003. 285(4):E876–E888.24. Grobet L, Pirottin D, Farnir F, Poncelet D, Royo LJ, Brouwers B, Christians E, Desmecht D, Coignoul F, Kahn R, Georges M. Modulating skeletal muscle mass by postnatal, muscle-specific inactivation of the myostatin gene. Genesis. 2003. 35(4):227–238.25. Razani B, Zhang XL, Bitzer M, von Gersdorff G, Böttinger EP, Lisanti MP. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. J Biol Chem. 2001. 276(9):6727–6738.26. Ohsawa Y, Hagiwara H, Nakatani M, Yasue A, Moriyama K, Murakami T, Tsuchida K, Noji S, Sunada Y. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition. J Clin Invest. 2006. 116(11):2924–2934.27. Schlegel A, Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000. 275(28):21605–21617.28. Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996. 271(25):15160–15165.29. Cameron PL, Ruffin JW, Bollag R, Rasmussen H, Cameron RS. Identification of caveolin and caveolin-related proteins in the brain. J Neurosci. 1997. 17(24):9520–9535.30. Ikezu T, Ueda H, Trapp BD, Nishiyama K, Sha JF, Volonte D, Galbiati F, Byrd AL, Bassell G, Serizawa H, Lane WS, Lisanti MP, Okamoto T. Affinity-purification and characterization of caveolins from the brain: Differential expression of caveolin-1, -2, and -3 in brain endothelial and astroglial cell types. Brain Res. 1998. 804(2):177–192.31. Bu J, Bruckner SR, Sengoku T, Geddes JW, Estus S. Glutamate regulates caveolin expression in rat hippocampal neurons. J Neurosci Res. 2003. 72(2):185–190.32. Arvanitis DN, Wang H, Bagshaw RD, Callahan JW, Boggs JM. Membrane-associated estrogen receptor and caveolin-1 are present in central nervous system myelin and oligodendrocyte plasma membranes. J Neurosci Res. 2004. 75(5):603–613.33. Pette D, Staron RS. Mammalian skeletal muscle fiber type transition. Int Rev Cytol. 1997. 170:143–223.34. Talmadge RJ. Myosin heavy chain isoform expression following reduced neuromuscular activity: potential regulatory mechanisms. Muscle Nerve. 2000. 23(5):661–679.35. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003. 552(Pt 2):499–511.36. Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003. 35(1):1–8.37. Round JM, Barr FM, Moffat B, Jones DA. Fibre areas and histochemical fibre types in the quadriceps muscle of paraplegic subjects. J Neurol Sci. 1993. 116(2):207–211.38. Rochester L, Barron MJ, Chandler CS, Sutton RA, Miller S, Johnson MA. Influence of electrical stimulation of the tibialis anterior muscle in paraplegic subjects. 2. Morphological and histochemical properties. Paraplegia. 1995. 33(9):514–522.39. Martinez-Moreno M, Martinez-Ruiz A, Alvarez-Barrientos A, Gavilanes F, Lamas S, Rodriguez-Crespo I. Nitric oxide downregulates caveolin-3 levels through the interaction with myogenin, its transcription factor. J Biol Chem. 2007. 282(32):23044–23054.40. Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997. 83(1):59–66.41. Tong L, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001. 8(6):1046–1056.42. Demetrovics Z, Kurimay T. Exercise addiction: a literature review. Psychiatr Hung. 2008. 23(2):129–141.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in the Expression of Smooth Muscle Myosin Heavy Chain mRNA following Partial Bladder Obstruction or Spinal Cord Injury in Rat: A Preliminary Study
- The Effect of Sprint and Heavy Resistance Training on Muscle Strength, Endurance and Muscle Fiber Type
- A Nonsense C5797T (R1933X) Mutation of MYH9 Gene in a Family with May-Hegglin Anomaly
- Quantitative Analysis of Myosin Heavy Chain (MHC) mRNA expression in Thyropharyngeus muscle and Cricopharyngeus muscle in Rats
- Effects of Treadmill Exercise on the Recovery of Dopaminergic Neuron Loss and Muscle Atrophy in the 6-OHDA Lesioned Parkinson's Disease Rat Model