Korean J Hematol.
2007 Sep;42(3):264-275. 10.5045/kjh.2007.42.3.264.
Generation and Qualification of Functionally Active Leukemia-derived DCs from Malignant Blasts in Acute Leukemia
- Affiliations
-
- 1The Cancer Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hlee@smc.samsung.co.kr
- 2Division of Hematology and Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2305202
- DOI: http://doi.org/10.5045/kjh.2007.42.3.264
Abstract
-
BACKGROUND: Dendritic cells (DCs) are increasingly being utilized for anti-cancer immunotherapy. Acute myeloid leukemia (AML) blasts are able to generate leukemia-derived DC. Advances in culture techniques and AML-DC characterization justify possible clinical applications. We investigated the ability of AML, acute lymphoblastic leukemia (ALL) and biphenotypic acute leukemia (BAL) blasts to differentiate into DCs in vitro and the qualified function of the leukemia-derived DCs.
METHODS
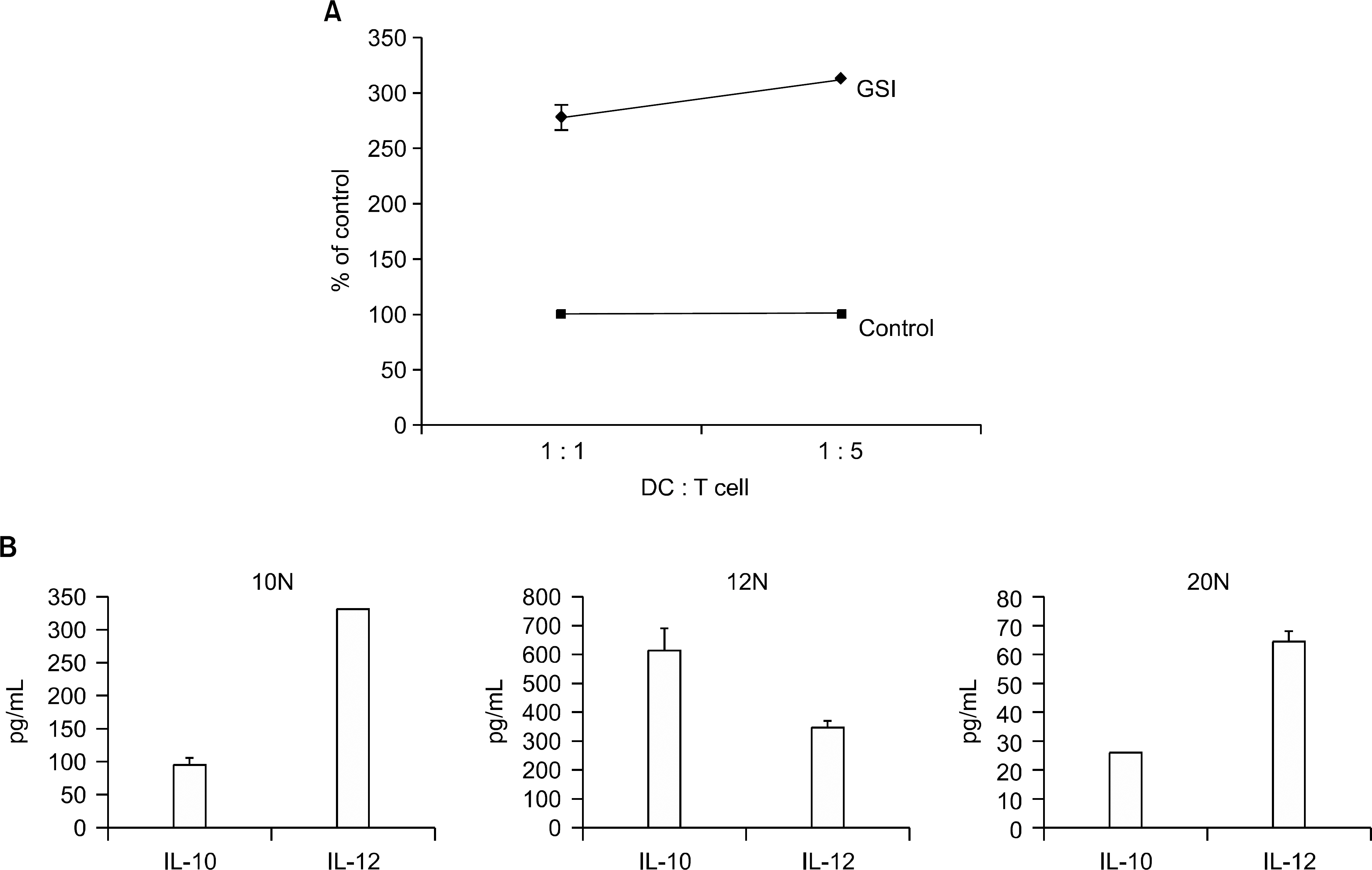
Leukemia cells from 11 patients with AML, 3 patients with ALL and 2 patients with BAL were cultured with GM-CSF, IL-4 and with or without SCF. Cultured leukemia cells were evaluated by phenotype, mixed lymphocyte reaction (MLR), cytokine production and cytotoxic T cell (CTL) inducing activity.
RESULTS
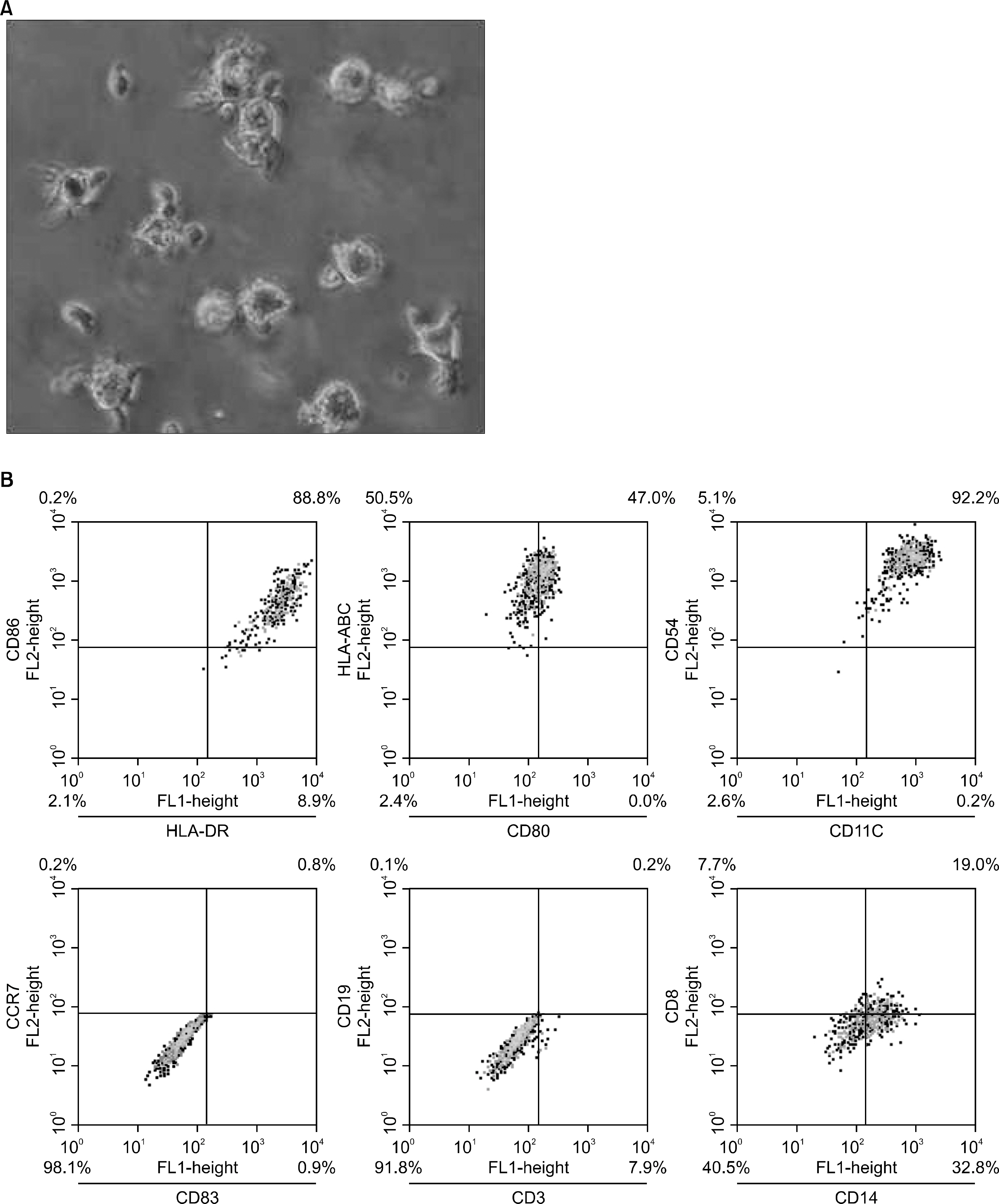
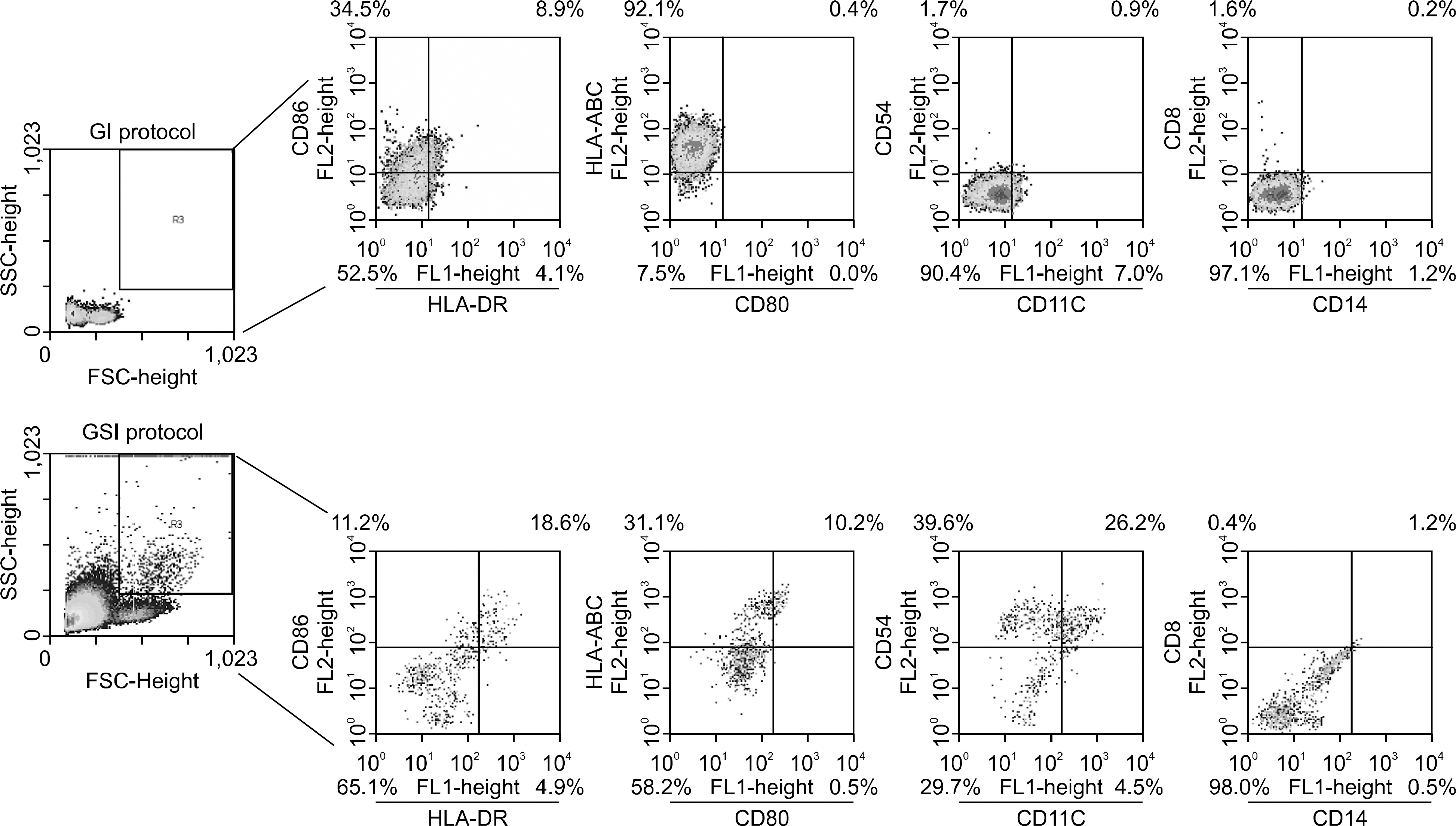
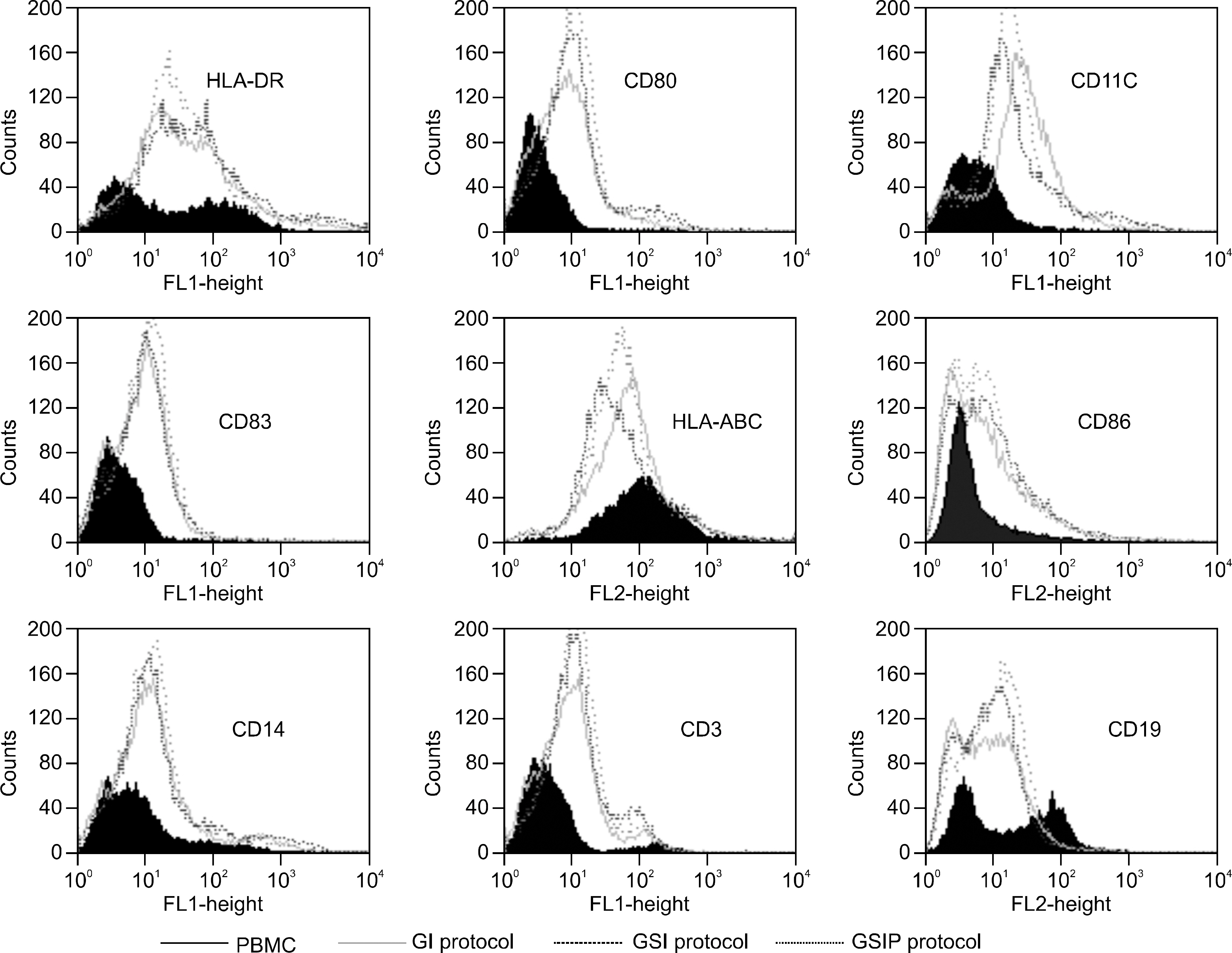
DCs were generated with GM-CSF and IL-4 from the leukemic blasts in 72% of the AML patient cells. MHC class I/II, CD11c and ICAM-1 were highly expressed in the AML-derived DCs. MLR and enzyme linked immunospot (ELISPOT) assays demonstrated that AML-DCs were able to induce T cell proliferation and activation into IFN-gamma secreting effector cells. The ALL blasts from two out of three patients differentiated into DCs with MHC class I/II+, CD11c+ only in the presence of GM-CSF, SCF and IL-4 for 14 days.
CONCLUSION
These results suggest that functionallyactive DCs can be differentiated from AML blasts using GM-GSF and IL-4 and ALL, BAL blasts were differentiated into DCs only under stem cell-DC culture conditions.
MeSH Terms
-
Cell Proliferation
Culture Techniques
Dendritic Cells
Granulocyte-Macrophage Colony-Stimulating Factor
Humans
Immunotherapy
Intercellular Adhesion Molecule-1
Interleukin-4
Leukemia*
Leukemia, Biphenotypic, Acute
Leukemia, Myeloid, Acute
Lymphocyte Culture Test, Mixed
Phenotype
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Granulocyte-Macrophage Colony-Stimulating Factor
Intercellular Adhesion Molecule-1
Interleukin-4
Figure
Reference
-
1). Steinman RM. Prospects for immunotheraphy directed to the T cell receptor in human autoimmune disease. Ann N Y Acad Sci. 1991. 636:147–53.2). Palucka K., Banchereau J. Dendritic cell: a link between innate and adaptive immunity. J Clin Immu-nol. 1999. 19:12–25.3). Celluzzi CM., Mayordomo JI., Storkus WJ., Lotze MT., Falo LD Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996. 183:283–7.
Article4). Thurner B., Haendle I., Röder C, et al. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999. 190:1669–78.
Article5). Lau R., Wang F., Jeffery G, et al. Phase I trial of intravenous peptide-pulsed dendritic cells in patients with metastatic melanoma. J Immunother. 2001. 24:66–78.
Article6). Geiger JD., Hutchinson RJ., Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001. 61:8513–9.7). Choudhury BA., Liang JC., Thomas EK, et al. Dendritic cells derived in vitro from acute myelogenous leukemia cells stimulate autologous, antileukemic T-cell responses. Blood. 1999. 93:780–6.
Article8). Harrison BD., Adams JA., Briggs M., Brereton ML., Yin JA. Stimulation of autologous proliferative and cytotoxic T-cell responses by leukemic dendritic cells derived from blast cells in acute myeloid leukemia. Blood. 2001. 97:2764–71.
Article9). Smith M., Barnett M., Bassan R., Gatta G., Tondini C., Kern W. Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004. 50:197–222.
Article10). Galea-Lauri J. Immunological weapons against acute myeloid leukaemia. Immunology. 2002. 107:20–7.
Article11). Lee JJ., Kook H., Park MS, et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J Clin Apher. 2004. 19:66–70.
Article12). Kemp R., Ronchese F. Tumor specific Tc1, but not Tc2, cells deliver protective antitumor immunity. J immunol. 2001. 167:6497–502.13). Barrett AJ., Horowitz MM., Ash RC, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992. 79:3067–70.
Article14). Blair A., Goulden NJ., Libri NA., Oakhill A., Pamphil-on DH. Immunotherapeutic strategies in acute lymphoblastic leukaemia relapsing after stem cell transplantation. Blood Rev. 2005. 19:289–300.
Article15). Eibl B., Ebner S., Duba C, et al. Dendritic cells generated from blood precursors of chronic myelogenous leukemia patients carry the Philadelphia translocation and can induce a CML-specific primary cytotoxic T-cell response. Genes Chromosomes Cancer. 1997. 20:215–23.
Article16). Choudhury A., Gajewski JL., Liang JC, et al. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1997. 89:1133–42.
Article17). Choudhury A., Liang JC., Thomas EK, et al. Dendritic cells derived in vitro from acute myelogenous leu-kemia cells stimulate autologous, antileukemic T cell responses. Blood. 1999. 93:780–6.18). Charbonnier A., Gaugler B., Sainty D., Lafage- Pochi-taloff M., Olive D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur J Immunol. 1999. 29:2567–78.19). Köhler T., Plettig R., Wetzstein W, et al. Cytokine-driven differentiation of blasts from patients with acute myelogenous and lymphoblastic leukemia into dendritic cells. Stem Cells. 2000. 18:139–47.
Article20). Engels FH., Koski GK., Bedrosian I, et al. Calcium signaling induces acquisition of dendritic cell characteristics in chronic myelogenous leukemia myeloid progenitor cells. Proc Natl Acad Sci USA. 1999. 96:10332–7.
Article21). Weigel BJ., Nath N., Taylor PA, et al. Comparative analysis of murine marrow-derived dendritic cells generated by Flt3L or GM-CSF/IL-4 and matured with immune stimulatory agents on the in vivo induction of antileukemia responses. Blood. 2002. 100:4169–76.
Article22). Ossenkoppele GJ., Stam AG., Westers TM, et al. Vaccination of chronic myeloid leukemia patients with autologous in vitro cultured leukemic dendritic cells. Leukemia. 2003. 17:1424–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Generation of Leukemic Dendritic Cells from Patients with Acute Myelogenous Leukemia
- A case of leukemia cutis in acute megakaryoblastic leukemia
- A Case of Acute Myeloid Leukemia with Multilineage Dysplasia accompanying Malignant Pleural Effusion
- A case report of adult acute biphenotypic leukemia-hand mirror variant
- Acute Megakaryoblastic Leukemia with CD41a-/CD61-/CD42a+ Blasts in an Infant with Down Syndrome