J Gynecol Oncol.
2011 Jun;22(2):110-119. 10.3802/jgo.2011.22.2.110.
Combined effect of CYP1B1, COMT, GSTP1, and MnSOD genotypes and risk of postmenopausal breast cancer
- Affiliations
-
- 1Institute of Medical Genetics, Department of Obstetrics and Gynecology, University Medical Center Ljubljana, Slajmerjeva 3, 1000 Ljubljana, Slovenia. ksenija.gersak@mf.uni-lj.si
- 2Institute for Biostatistics and Medical Informatics, Faculty of Medicine, University of Ljubljana, Vrazov trg 2, 1000 Ljubljana, Slovenia.
- 3Department of Molecular Diagnostics, Institute of Oncology Ljubljana, Zaloska 2, 1000 Ljubljana, Slovenia.
- 4Department of Pathology, Institute of Oncology Ljubljana, Zaloska 2, 1000 Ljubljana, Slovenia.
- KMID: 2288575
- DOI: http://doi.org/10.3802/jgo.2011.22.2.110
Abstract
OBJECTIVE
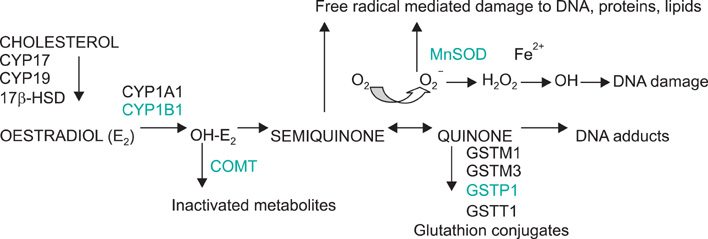
Estrogen plays a key role in breast cancer development and functionally relevant genetic variants within the estrogen metabolic pathway are prime candidates for a possible association with breast cancer risk. We investigated the independent and the combined effects of commonly occurring polymorphisms in four genes encoding key proteins of estrogen metabolic pathway on their potential contribution to breast cancer risk.
METHODS
We studied 530 breast cancer cases and 270 controls of the same age and ethnicity participating in a case-control study of postmenopausal women. Genotyping was conducted for CYP1B1 (rs1056836), COMT (rs4680), GSTP1 (rs1695), and MnSOD (rs4880) polymorphisms by polymerase chain reaction based restriction fragment length polymorphism and TaqMan allelic discrimination method. Adjusted ORs and 95% CIs were calculated using logistic regression.
RESULTS
None of the 4 genetic variants examined contributed to breast cancer risk individually. When the combined effects of the risk genotypes were investigated, significant associations were observed among women with two high-risk genotypes in CYP1B1 and COMT (OR, 2.0; 95% CI, 1.1 to 3.5) and two high-risk genotypes in COMT and MnSOD (OR, 2.0; 95% CI, 1.0 to 3.8), compared to those with low-risk genotypes.
CONCLUSION
Our results suggest that individual susceptibility to breast cancer incidence may be increased by combined effects of the high-risk genotypes in CYP1B1, COMT, and MnSOD estrogen metabolic genes.
MeSH Terms
-
Aryl Hydrocarbon Hydroxylases
Breast
Breast Neoplasms
Case-Control Studies
Discrimination (Psychology)
Electrolytes
Estrogens
Female
Genotype
Humans
Incidence
Metabolic Networks and Pathways
Polymerase Chain Reaction
Polymorphism, Restriction Fragment Length
Proteins
Aryl Hydrocarbon Hydroxylases
Electrolytes
Estrogens
Proteins
Figure
Reference
-
1. Hunter DJ, Riboli E, Haiman CA, Albanes D, Altshuler D, Chanock SJ, et al. A candidate gene approach to searching for low-penetrance breast and prostate cancer genes. Nat Rev Cancer. 2005. 5:977–985.2. Pharoah PD, Dunning AM, Ponder BA, Easton DF. Association studies for finding cancer-susceptibility genetic variants. Nat Rev Cancer. 2004. 4:850–860.3. Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006. 354:270–282.4. Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003. 95:1218–1226.5. Key T, Appleby P, Barnes I, Reeves G. Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002. 94:606–616.6. Onland-Moret NC, Kaaks R, van Noord PA, Rinaldi S, Key T, Grobbee DE, et al. Urinary endogenous sex hormone levels and the risk of postmenopausal breast cancer. Br J Cancer. 2003. 88:1394–1399.7. Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004. 96:1856–1865.8. Yue W, Santen RJ, Wang JP, Li Y, Verderame MF, Bocchinfuso WP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J Steroid Biochem Mol Biol. 2003. 86:477–486.9. Cavalieri E, Frenkel K, Liehr JG, Rogan E, Roy D. Estrogens as endogenous genotoxic agents: DNA adducts and mutations. J Natl Cancer Inst Monogr. 2000. (27):75–93.10. Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000. (27):67–73.11. Ball P, Knuppen R. Catecholoestrogens (2-and 4-hydroxyoestrogens): chemistry, biogenesis, metabolism, occurrence and physiological significance. Acta Endocrinol Suppl (Copenh). 1980. 232:1–127.12. Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer: the role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat Res. 2003. 544:9–41.13. Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci U S A. 1996. 93:9776–9781.14. Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr Rev. 2000. 21:40–54.15. Raftogianis R, Creveling C, Weinshilboum R, Weisz J. Estrogen metabolism by conjugation. J Natl Cancer Inst Monogr. 2000. (27):113–124.16. Forrester LM, Hayes JD, Millis R, Barnes D, Harris AL, Schlager JJ, et al. Expression of glutathione S-transferases and cytochrome P450 in normal and tumor breast tissue. Carcinogenesis. 1990. 11:2163–2170.17. Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995. 64:97–112.18. Tworoger SS, Chubak J, Aiello EJ, Ulrich CM, Atkinson C, Potter JD, et al. Association of CYP17, CYP19, CYP1B1, and COMT polymorphisms with serum and urinary sex hormone concentrations in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2004. 13:94–101.19. Dunning AM, Dowsett M, Healey CS, Tee L, Luben RN, Folkerd E, et al. Polymorphisms associated with circulating sex hormone levels in postmenopausal women. J Natl Cancer Inst. 2004. 96:936–945.20. Westfall PH, Young SS. Resampling-based multiple testing. 1993. New York: John Wiley & Sons Inc..21. Pharoah PD, Tyrer J, Dunning AM, Easton DF, Ponder BA. SEARCH Investigators. Association between common variation in 120 candidate genes and breast cancer risk. PLoS Genet. 2007. 3:e42.22. Colhoun HM, McKeigue PM, Davey Smith G. Problems of reporting genetic associations with complex outcomes. Lancet. 2003. 361:865–872.23. Kocabas NA, Sardas S, Cholerton S, Daly AK, Elhan AH, Karakaya AE. Genetic polymorphism of manganese superoxide dismutase (MnSOD) and breast cancer susceptibility. Cell Biochem Funct. 2005. 23:73–76.24. Li DN, Seidel A, Pritchard MP, Wolf CR, Friedberg T. Polymorphisms in P450 CYP1B1 affect the conversion of estradiol to the potentially carcinogenic metabolite 4-hydroxyestradiol. Pharmacogenetics. 2000. 10:343–353.25. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996. 6:243–250.26. Zimniak P, Nanduri B, Pikula S, Bandorowicz-Pikula J, Singhal SS, Srivastava SK, et al. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994. 224:893–899.27. Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003. 13:145–157.28. Bag A, Bag N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev. 2008. 17:3298–3305.29. Hu X, Xia H, Srivastava SK, Pal A, Awasthi YC, Zimniak P, et al. Catalytic efficiencies of allelic variants of human glutathione S-transferase P1-1 toward carcinogenic antidiol epoxides of benzo[c]phenanthrene and benzo[g]chrysene. Cancer Res. 1998. 58:5340–5343.30. Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, et al. Glutathione S-transferase M1, M3, P1, and T1 genetic polymorphisms and susceptibility to breast cancer. Cancer Epidemiol Biomarkers Prev. 2001. 10:229–236.31. Helzlsouer KJ, Selmin O, Huang HY, Strickland PT, Hoffman S, Alberg AJ, et al. Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst. 1998. 90:512–518.32. Park SK, Yim DS, Yoon KS, Choi IM, Choi JY, Yoo KY, et al. Combined effect of GSTM1, GSTT1, and COMT genotypes in individual breast cancer risk. Breast Cancer Res Treat. 2004. 88:55–62.33. Mitrunen K, Kataja V, Eskelinen M, Kosma VM, Kang D, Benhamou S, et al. Combined COMT and GST genotypes and hormone replacement therapy associated breast cancer risk. Pharmacogenetics. 2002. 12:67–72.34. Cheng TC, Chen ST, Huang CS, Fu YP, Yu JC, Cheng CW, et al. Breast cancer risk associated with genotype polymorphism of the catechol estrogen-metabolizing genes: a multigenic study on cancer susceptibility. Int J Cancer. 2005. 113:345–353.35. Reding KW, Weiss NS, Chen C, Li CI, Carlson CS, Wilkerson HW, et al. Genetic polymorphisms in the catechol estrogen metabolism pathway and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009. 18:1461–1467.36. Millikan R, Pittman G, Tse CK, Savitz DA, Newman B, Bell D. Glutathione S-transferases M1, T1, and P1 and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000. 9:567–573.37. Curran JE, Weinstein SR, Griffiths LR. Polymorphisms of glutathione S-transferase genes (GSTM1, GSTP1 and GSTT1) and breast cancer susceptibility. Cancer Lett. 2000. 153:113–120.38. Onay VU, Briollais L, Knight JA, Shi E, Wang Y, Wells S, et al. SNP-SNP interactions in breast cancer susceptibility. BMC Cancer. 2006. 6:114.39. Rae JM, Cordero KE, Scheys JO, Lippman ME, Flockhart DA, Johnson MD. Genotyping for polymorphic drug metabolizing enzymes from paraffin-embedded and immunohistochemically stained tumor samples. Pharmacogenetics. 2003. 13:501–507.40. Kweekel DM, Van der Straaten T, Koopman M, Meijer GA, Nortier JW, Cornelis JA, et al. Comparison of genetic polymorphisms in DNA isolated from blood and paraffin embedded colorectal cancer tissue [Internet]. cited 2011 May 10. Available from: https://www.openaccess.leidenuniv.nl/bitstream/1887/13820/15/03.pdf.41. Xie B, Freudenheim JL, Cummings SS, Singh B, He H, McCann SE, et al. Accurate genotyping from paraffin-embedded normal tissue adjacent to breast cancer. Carcinogenesis. 2006. 27:307–310.42. Briollais L, Wang Y, Rajendram I, Onay V, Shi E, Knight J, et al. Methodological issues in detecting gene-gene interactions in breast cancer susceptibility: a population-based study in Ontario. BMC Med. 2007. 5:22.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Alanine-Valine Manganese Superoxide Dismutase Gene Polymorphism and Microheterogeneity Manganese Superoxide Dismutase Activity in Breast Cancer and Benign Breast Tissue
- Glutathione S-transferase P1 Genetic Polymorphisms and Breast Cancer Risk
- GSTP1 Polymorphism, Cigarette Smoking and Cervical Cancer Risk in Korean Women
- Relationship between Genetic Polymorphisms of the Gltathione S-transferase and Endometriosis Susceptibility in Korean Populations
- The Catechol-O-Methyltransferase Val158Met Polymorphism Contributes to the Risk of Breast Cancer in the Chinese Population: An Updated Meta-Analysis