J Clin Neurol.
2009 Jun;5(2):91-94. 10.3988/jcn.2009.5.2.91.
Scoliosis in Patients with Parkinson's Disease
- Affiliations
-
- 1Department of Neurology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 2Department of Neurology, Kangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. mslee@yuhs.ac
- KMID: 2287660
- DOI: http://doi.org/10.3988/jcn.2009.5.2.91
Abstract
-
BACKGROUND AND PURPOSE: Scoliosis is more common in patients with Parkinson's disease (PD) than in the general elderly population. We compared clinical characteristics between PD patients with and without scoliosis, to identify the relationship between the direction of scoliosis and the laterality of the dominant symptoms of PD. We also studied the associations between dopaminergic pharmacotherapy and scoliosis (defined by a spinal curvature deviation of 10 degrees or larger).
METHODS
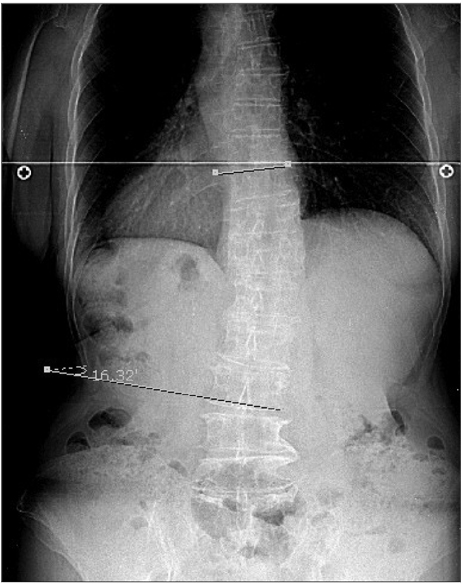
The study population comprised 97 patients (42 men and 55 women) with idiopathic PD. All of the patients submitted to a whole-spine scanograph to allow measurement of the degree of scoliosis by Cobb's method.
RESULTS
True scoliosis was found in 32 of the 97 PD patients, and was observed more frequently in women than in men (28 vs. 4, respectively; p=0.006). The age of patients without scoliosis was significantly lower than that of those with scoliosis (66.5+/-9.2 years vs. 72.8+/-7.3 years, respectively, mean+/-SD, p<0.001). There was no correlation between PD symptom laterality and scoliosis. The rate of occurrence of scoliosis did not differ between de novo and levodopa (L-dopa)-treated patients.
CONCLUSIONS
We suggest that neither L-dopa treatment nor the laterality of the initial symptoms of PD is related to the appearance of scoliosis.
Keyword
Figure
Reference
-
1. Robin GC, Span Y, Steinberg R, Makin M, Menczel J. Scoliosis in the elderly: a follow-up study. Spine. 1982. 7:355–359.2. Vanderpool DW, James JI, Wynne-Davies R. Scoliosis in the elderly. J Bone Joint Surg AM. 1969. 51:446–455.
Article3. Duvoisin RC, Marsden CD. Note on the scoliosis of Parkinsonism. J Neurol Neurosurg Psychiatry. 1975. 38:787–793.
Article4. Indo T, Ando K. [Studies on the scoliosis of Parkinsonism (author's transl)]. Rinsho Shin-keigaku. 1980. 20:40–46.5. Martin JP. Curvature of the spine in post-encephalitic parkinsonism. J Neurol Neurosurg Psychiatry. 1965. 28:395–400.
Article6. Herrera-Marschitz M, Utsumi H, Ungerstedt U. Scoliosis in rats with experimentally-induced hemiparkinsonism: dependence upon striatal dopamine denervation. J Neurol Neurosurg Psychiatry. 1990. 53:39–43.
Article7. Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004. 16:110–123.
Article8. Grimes JD, Hassan MN, Trent G, Halle D, Armstrong GW. Clinical and radiographic features of scoliosis in Parkinson's disease. Adv Neurol. 1987. 45:353–355.9. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992. 42:1142–1146.
Article10. Fahn S, Elton RL. Fahn S, Marsden DB, Calne CD, Golstein M, editors. Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. Recent developments in Parkinson's disease. 1987. Vol. 2. Florham Park (NJ): Macmillan Health Care;153–164.11. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967. 17:427–442.
Article12. Cobb JR. Outline for the study of scoliosis. Am Acad Orthop Surg. 1948. 5:261–275.13. Van Goethem J, Van Campenhout A, van den Hauwe L, Parizel PM. Scoliosis. Neuroimaging Clin N Am. 2007. 17:105–115.
Article14. Ashour R, Jankovic J. Joint and skeletal deformities in Parkinson's disease, multiple system atrophy, and progressive supranuclear palsy. Mov Disord. 2006. 21:1856–1863.
Article15. Lowe TG, Edgar M, Margulies JY, Miller NH, Raso VJ, Reinker KA, et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg Am. 2000. 82-A:1157–1168.
Article16. Jankovic J, Tintner R. Dystonia and parkinsonism. Parkinsonism Relat Disord. 2001. 8:109–121.
Article17. Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971. 367:69–93.
Article18. Péchadre JC, Larochelle L, Poirier LJ. Parkinsonian akinesia, rigidity and tremor in the monkey. Histopathological and neuropharmacological study. J Neurol Sci. 1976. 28:147–157.19. Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop. 2003. 32:77–82. discussion 82.20. Healey JH, Lane JM. Structural scoliosis in osteoporotic women. Clin Orthop Relat Res. 1985. 216–223.
Article21. Robin GC. Scoliosis in the elderly: idiopathic or osteoporotic? Clin Orthop Relat Res. 1986. 311–312.22. Thevenon A, Pollez B, Cantegrit F, Tison-Muchery F, Marchandise X, Duquesnoy B. Relationship between kyphosis, scoliosis, and osteoporosis in the elderly population. Spine. 1987. 12:744–745.
Article