Korean J Physiol Pharmacol.
2016 May;20(3):245-251. 10.4196/kjpp.2016.20.3.245.
Prospective validation of a novel dosing scheme for intravenous busulfan in adult patients undergoing hematopoietic stem cell transplantation
- Affiliations
-
- 1Department of Clinical Pharmacology, Inha University Hospital, Inha University School of Medicine, Incheon 22332, Korea.
- 2Department of Hematology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 3Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. ksbae@amc.seoul.kr
- 4Department of Clinical Pharmacology and Therapeutics, Pusan National University Hospital, Busan 49241, Korea.
- KMID: 2285596
- DOI: http://doi.org/10.4196/kjpp.2016.20.3.245
Abstract
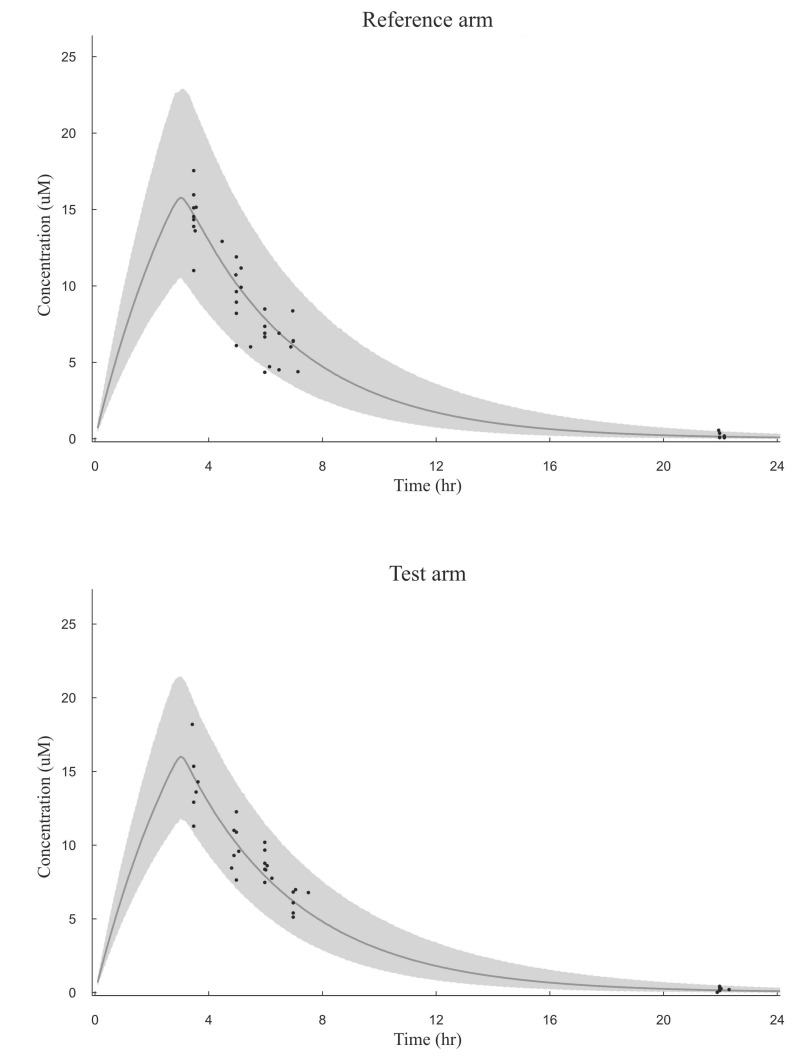
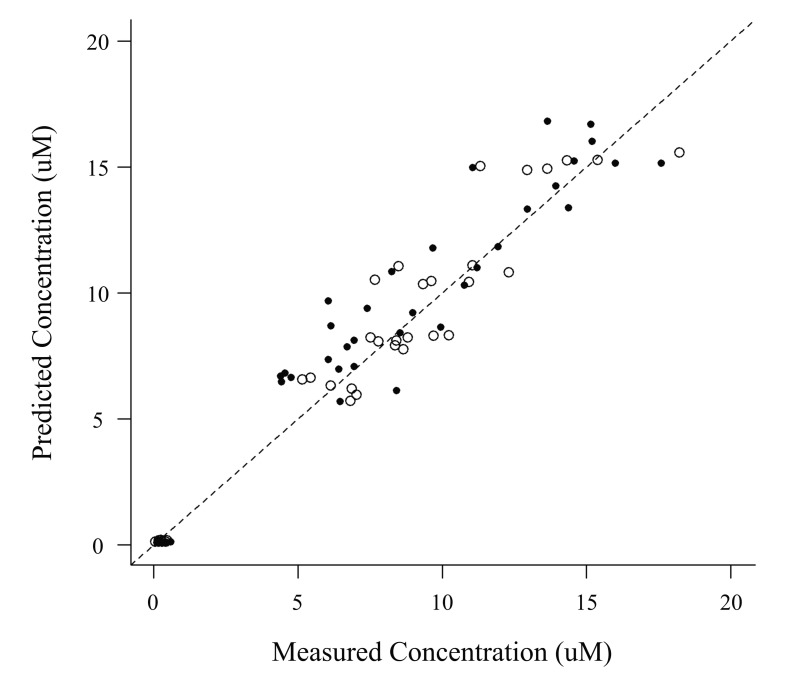
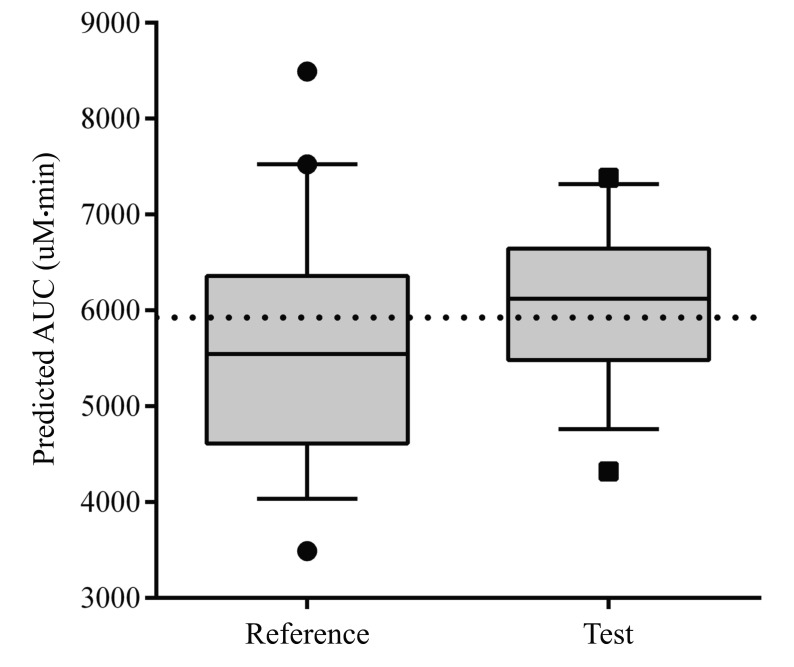
- The objective of this study was to externally validate a new dosing scheme for busulfan. Thirty-seven adult patients who received busulfan as conditioning therapy for hematopoietic stem cell transplantation (HCT) participated in this prospective study. Patients were randomized to receive intravenous busulfan, either as the conventional dosage (3.2 mg/kg daily) or according to the new dosing scheme based on their actual body weight (ABW) (23×ABW(0.5) mg daily) targeting an area under the concentration-time curve (AUC) of 5924 µM·min. Pharmacokinetic profiles were collected using a limited sampling strategy by randomly selecting 2 time points at 3.5, 5, 6, 7 or 22 hours after starting busulfan administration. Using an established population pharmacokinetic model with NONMEM software, busulfan concentrations at the available blood sampling times were predicted from dosage history and demographic data. The predicted and measured concentrations were compared by a visual predictive check (VPC). Maximum a posteriori Bayesian estimators were estimated to calculate the predicted AUC (AUC(PRED)). The accuracy and precision of the AUC(PRED) values were assessed by calculating the mean prediction error (MPE) and root mean squared prediction error (RMSE), and compared with the target AUC of 5924 µM·min. VPC showed that most data fell within the 95% prediction interval. MPE and RMSE of AUCPRED were -5.8% and 20.6%, respectively, in the conventional dosing group and −2.1% and 14.0%, respectively, in the new dosing scheme group. These fi ndings demonstrated the validity of a new dosing scheme for daily intravenous busulfan used as conditioning therapy for HCT.
Keyword
MeSH Terms
Figure
Reference
-
1. Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, Braine HG, Burns WH, Elfenbein GJ, Kaizer H, Mellits D, Sensenbrenner LL, Stuart RK, Yeager AM. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med. 1983; 309:1347–1353. PMID: 6355849.
Article2. Parkman R, Rappeport JM, Hellman S, Lipton J, Smith B, Geha R, Nathan DG. Busulfan and total body irradiation as antihematopoietic stem cell agents in the preparation of patients with congenital bone marrow disorders for allogenic bone marrow transplantation. Blood. 1984; 64:852–857. PMID: 6383499.
Article3. Blazar BR, Ramsay NK, Kersey JH, Krivit W, Arthur DC, Filipovich AH. Pretransplant conditioning with busulfan (Myleran) and cyclophosphamide for nonmalignant diseases. Assessment of engraftment following histocompatible allogeneic bone marrow transplantation. Transplantation. 1985; 39:597–603. PMID: 3890287.4. Lucarelli G, Polchi P, Izzi T, Manna M, Delfini C, Galimberti M, Porcellini A, Moretti L, Manna A, Sparaventi G. Marrow transplantation for thalassemia after treatment with busulfan and cyclophosphamide. Ann N Y Acad Sci. 1985; 445:428–431. PMID: 3893278.
Article5. Hartman AR, Williams SF, Dillon JJ. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: a meta-analysis. Bone Marrow Transplant. 1998; 22:439–443. PMID: 9733266.6. Bleyzac N, Souillet G, Magron P, Janoly A, Martin P, Bertrand Y, Galambrun C, Dai Q, Maire P, Jelliffe RW, Aulagner G. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001; 28:743–751. PMID: 11781625.
Article7. Schuler US, Ehrsam M, Schneider A, Schmidt H, Deeg J, Ehninger G. Pharmacokinetics of intravenous busulfan and evaluation of the bioavailability of the oral formulation in conditioning for haematopoietic stem cell transplantation. Bone Marrow Transplant. 1998; 22:241–244. PMID: 9720736.
Article8. Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R, Santos GW, Colvin OM. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol. 1989; 25:55–61. PMID: 2591002.
Article9. Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, Lin LS, Devine SM, Geller RB, Heffner LT, Hillyer CD, Holland HK, Winton EF, Saral R. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996; 17:225–230. PMID: 8640171.10. Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, Soll E, Anasetti C, Bowden R, Bryant E, Chauncey T, Deeg HJ, Doney KC, Flowers M, Gooley T, Hansen JA, Martin PJ, McDonald GB, Nash R, Petersdorf EW, Sanders JE, Schoch G, Stewart P, Storb R, Sullivan KM, Thomas ED, Witherspoon RP, Appelbaum FR. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997; 89:3055–3060. PMID: 9108427.
Article11. Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, Blume K, Niland J, Palmer JM, Vaughan W, Fernandez H, Champlin R, Forman S, Andersson BS. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002; 8:493–500. PMID: 12374454.
Article12. Zwaveling J, Bredius RG, Cremers SC, Ball LM, Lankester AC, Teepe-Twiss IM, Egeler RM, den Hartigh J, Vossen JM. Intravenous busulfan in children prior to stem cell transplantation: study of pharmacokinetics in association with early clinical outcome and toxicity. Bone Marrow Transplant. 2005; 35:17–23. PMID: 15502853.
Article13. Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant. 2002; 8:486–492. PMID: 12374453.
Article14. Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, Stewart D, Ruether JD, Morris D, Glick S, Gyonyor E, Andersson BS. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002; 8:468–476. PMID: 12374451.
Article15. Ryu SG, Lee JH, Choi SJ, Lee YS, Seol M, Hur EH, Lee SH, Bae KS, Noh GJ, Lee MS, Yun SC, Han SB, Lee KH. Randomized comparison of four-times-daily versus once-daily intravenous busulfan in conditioning therapy for hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007; 13:1095–1105. PMID: 17697972.
Article16. Bartelink IH, Bredius RG, Ververs TT, Raphael MF, van Kesteren C, Bierings M, Rademaker CM, den Hartigh J, Uiterwaal CS, Zwaveling J, Boelens JJ. Once-daily intravenous busulfan with therapeutic drug monitoring compared to conventional oral busulfan improves survival and engraftment in children undergoing allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2008; 14:88–98.
Article17. Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, Savoie ML, Bahlis NJ, Brown C, Storek J, Andersson BS, Russell JA. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008; 14:220–228. PMID: 18215782.
Article18. Choe S, Kim G, Lim HS, Cho SH, Ghim JL, Jung JA, Kim UJ, Noh G, Bae KS, Lee D. A simple dosing scheme for intravenous busulfan based on retrospective population pharmacokinetic analysis in korean patients. Korean J Physiol Pharmacol. 2012; 16:273–280. PMID: 22915993.
Article19. Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997; 37:486–495. PMID: 9208355.
Article20. Brendel K, Comets E, Laffont C, Laveille C, Mentre F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm Res. 2006; 23:2036–2049. PMID: 16906454.
Article21. Mentre F, Escolano S. Prediction discrepancies for the evaluation of nonlinear mixed-effects models. J Pharmacokinet Pharmacodyn. 2006; 33:345–367. PMID: 16284919.
Article22. Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007; 46:221–234. PMID: 17328581.23. Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)--a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004; 75:85–94. PMID: 15212851.
Article24. Grochow LB. Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol. 1993; 20:18–25. quiz 2625. Ljungman P, Hassan M, Bekassy AN, Ringden O, Oberg G. High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant. 1997; 20:909–913. PMID: 9422468.
Article26. Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y. Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2006; 37:345–351. PMID: 16400337.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Simple Dosing Scheme for Intravenous Busulfan Based on Retrospective Population Pharmacokinetic Analysis in Korean Patients
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Outcome of Hematopoietic Stem Cell Transplantation in Wiskott-Aldrich Syndrome
- The Effectiveness of Once-daily Intravenous Busulfan as a Conditioning Regime for Hematopoietic Stem Cell Transplantation in Children with Acute Myelogenous Leukemia
- Hematopoietic Stem Cell Transplantation